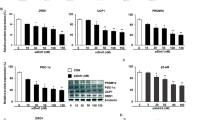
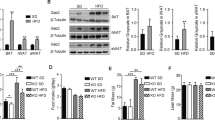
Abstract
ADAMTSs belong to the superfamily of secreted metalloendopeptidases, some of which are reported to be closely associated with obesity. However, the role of ADAMTS15 is not well characterized in adipocytes. This study investigates the effect of Adamts15 deficiency on lipid metabolism in 3T3-L1 and HIB1B adipocytes, with a focus on the role of browning of white adipocytes. Quantitative real-time PCR, immunoblot analysis, immunofluorescence, and staining methods were applied to evaluate the effects of ADAMTS15 on other target proteins and genes involved in lipid metabolism, after silencing Adamts15 by applying the siRNA technique. Our results demonstrate that ADAMTS15 is expressed in both white and brown adipocytes, and the deficiency of Adamts15 promotes browning of white adipocytes by enhancing the expression of brown adipocyte-specific genes and proteins. In addition, silencing of Adamts15 activates brown adipocytes and also upregulates lipid metabolic activity in both white and brown adipocytes, by increasing mitochondrial biogenesis as well as the levels of lipolytic and fat oxidative marker proteins, and reducing adipogenic factors. Moreover, mechanistic studies revealed that depletion of Adamts15 induces browning via activation of the β3AR-PKA-CREB signaling pathway. Taken together, our data unveiled a previously unknown mechanism of ADAMTS15 in the regulation of lipids, and the significance of this protein as a pharmacotherapeutic target to treat obesity-related metabolic disorders.
Similar content being viewed by others
Abbreviations
- ACC :
-
acyl-CoA carboxylase
- ACO :
-
acyl-coenzyme A oxidase 1
- ADAMTS/Adamts :
-
a disintegrin and metallo-proteinase with thrombospondin motifs/encoding gene
- AMPK :
-
AMP-activated protein kinase
- AR :
-
adrenergic receptor
- ATF :
-
activating transcription factor
- ATGL :
-
adipose triglyceride lipase
- Cd137 :
-
tumor necrosis factor receptor superfamily, member 9
- Cited1 :
-
gene encoding Cbp/p300-interacting transactivator 1
- C/EBP/Cebp :
-
CCAAT/enhancer-binding protein/encoding gene
- CREB :
-
cyclic AMP response element-binding protein
- CPT1 :
-
carnitine palmitoyl transferase 1
- ERK :
-
extracellular-signal-regulated kinase
- HSL :
-
hormone-sensitive lipase
- FGF21 :
-
fibroblast growth factor 21
- mTORC1 :
-
mammalian target of rapamycin complex 1
- PDE4 :
-
phosphodiesterase 4
- PGC-1α /Ppargc1α :
-
peroxisome proliferator-activated receptor gamma co-activator 1-alpha/encoding gene
- Nrf1 :
-
nuclear respiratory factor 1-encoding gene
- PKA :
-
protein kinase A
- PPAR :
-
peroxisome proliferator-activated receptor
- PRDM16/Prdm16 :
-
PR domain-containing 16/encoding gene
- p38 MAPK :
-
mitogen-activated protein kinase 14
- Tbx1 :
-
gene encoding T-box protein 1
- Tfam :
-
gene encoding mitochondrial transcription factor
- Tmem26 :
-
gene encoding transmembrane protein 26
- UCP1/Ucp1 :
-
uncoupling protein 1/encoding gene
- VEGF :
-
vascular endothelial growth factor
References
Nicol, K. K., B. J. Shelton, M. A. Knovich, and J. Owen (2003) Overweight individuals are at increased risk for thrombotic thrombocytopenic purpura. Am. J. Hematol. 74: 170–174.
Deford, C. C., J. A. Reese, L. H. Schwartz, J. J. Perdue, J. A. Kremer Hovinga, B. Lammle, D. R. Terrell, S. K. Vesely, and J. N. George (2013) Multiple major morbidities and increased mortality during long-term follow-up after recovery from thrombotic thrombocytopenic purpura. Blood. 122: 2023–2029.
Geys, L., I. Scroyen, E. Roose, K. Vanhoorelbeke, and H. R. Lijnen (2015) ADAMTS13 deficiency in mice does not affect adipose tissue development. Biochim. Biophys. Acta. 1850: 1368–1374.
Rocks, N., G. Paulissen, M. El Hour, F. Quesada, C. Crahay, M. Gueders, J. M. Foidart, A. Noel, and D. Cataldo (2008) Emerging roles of ADAM and ADAMTS metalloproteinases in cancer. Biochimie. 90: 369–379.
Kong, P., C. Gonzalez-Quesada, N. Li, M. Cavalera, D. W. Lee, and N. G. Frangogiannis (2013) Thrombospondin-1 regulates adiposity and metabolic dysfunction in diet-induced obesity enhancing adipose inflammation and stimulating adipocyte proliferation. Am. J. Physiol. Endocrinol. Metab. 305: E439–E450.
Porter, S., I. M. Clark, L. Kevorkian, and D. R. Edwards (2005) The ADAMTS metalloproteinases. Biochem. J. 386: 15–27.
Mead, T. J. and S. S. Apte (2018) ADAMTS proteins in human disorders. Matrix Biol. 71–72: 225–239.
Christiaens, V., I. Scroyen, and H. R. Lijnen (2008) Role of proteolysis in development of murine adipose tissue. Thromb. Haemost. 99: 290–294.
Sun, K., C. M. Kusminski, and P. E. Scherer (2011) Adipose tissue remodeling and obesity. J. Clin. Invest. 121: 2094–2101.
Dubail, J. and S. S. Apte (2015) Insights on ADAMTS proteases and ADAMTS-like proteins from mammalian genetics. Matrix Biol. 44–46: 24–37.
Kelwick, R., I. Desanlis, G. N. Wheeler, and D. R. Edwards (2015) The ADAMTS (A disintegrin and metalloproteinase with thrombospondin motifs) family. Genome Biol. 16: 113.
Mariman, E. C. M. and P. Wang (2010) Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell Mol. Life Sci. 67: 1277–1292.
Bonnans, C., J. Chou, and Z. Werb (2014) Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 15: 786–801.
Voros, G., J. D. Sandy, D. Collen, and H. R. Lijnen (2006) Expression of aggrecan(ases) during murine preadipocyte differentiation and adipose tissue development. Biochim. Biophys. Acta. 1760: 1837–1844.
Crawley, J. T. B., D. A. Lane, M. Woodward, A. Rumley, and G. D. O. Lowe (2008) Evidence that high von Willebrand factor and low ADAMTS-13 levels independently increase the risk of a non-fatal heart attack. J. Thromb. Haemost. 6: 583–588.
Liu, M. Y., Z. Zhou, R. Ma, Z. Tao, H. Choi, A. L. Bergeron, H. Wu, and J. Dong (2012) Gender-dependent up-regulation of ADAMTS-13 in mice with obesity and hypercholesterolemia. Thromb. Res. 129: 536–539.
Pan, R., X. Zhu, P. Maretich, and Y. Chen (2020) Combating obesity with thermogenic fat: current challenges and advancements. Front. Endocrinol (Lausanne). 11: 185.
Jeremic, N., P. Chaturvedi, S. C. Tyagi (2017) Browning of white fat: novel insight into factors, mechanisms, and therapeutics. J. Cell Physiol. 232: 61–68.
Manigandan, S. and J. W. Yun (2020) Urolithin A induces brown-like phenotype in 3T3-L1 white adipocytes via β3-adrenergic receptor-p38 MAPK signaling pathway. Biotechnol. Bioprocess Eng. 25: 345–355.
Mukherjee, S., K. R. Aseer, and J. W. Yun (2020) Roles of macrophage colony stimulating factor in white and brown adipocytes. Biotechnol. Bioprocess Eng. 25: 29–38.
Bauters, D., M. Cobbaut, L. Geys, J. V. Lint, B. Hemmeryckx, and H. R. Lijnen (2017) Loss of ADAMTS5 enhances brown adipose tissue mass and promotes browning of white adipose tissue via CREB signaling. Mol. Metab. 6: 715–724.
Bauters, D., P. Bedossa, H. R. Lijnen, and B. Hemmeryckx (2018) Functional role of ADAMTS5 in adiposity and metabolic health. PLoS One. 13: e0190595.
Kurisaki, T., A. Masuda, K. Sudo, J. Sakagami, S. Higashiyama, Y. Matsuda, A. Nagabukuro, A. Tsuji, Y. Nabeshima, M. Asano, Y. Iwakura, and A. Sehara-Fujisawa (2003) Phenotypic analysis of meltrin a (ADAM12)-deficient mice: involvement of meltrin a in adipogenesis and myogenesis. Mol. Cell Biol. 23: 55–61.
Porter, S., P. N. Span, F. C. G. J. Sweep, V. C. G. Tjan-Heijnen, C. J. Pennington, T. X. Pedersen, M. Johnsen, L. R. Lund, J. Rømer, and D. R. Edwards (2006) ADAMTS8 and ADAMTS15 expression predicts survival in human breast carcinoma. Int. J. Cancer. 118: 1241–1247.
Binder, M. J., S. McCoombe, E. D. Williams, D. R. McCulloch, and A. C. Ward (2020) ADAMTS-15 has a tumor suppressor role in prostate cancer. Biomolecules. 10: 682.
Dimauro, I., T. Pearson, D. Caporossi, and M. J. Jackson (2012) A simple protocol for the subcellular fractionation of skeletal muscle cells and tissue. BMC Res. Notes 5: 513.
Bauters, D., P. SpI.incemaille, L. Geys, D. Cassiman, P. Vermeersch, P. Bedossa, I. Scroyen, and H. R. Lijnen (2016) ADAMTS5 deficiency protects against non-alcoholic steatohepatitis in obesity. Liver Int. 36: 1848–1859.
Bauters, D., I. Scroyen, R. Deprez-Poulain, and H. R. Lijnen (2016) ADAMTS5 promotes murine adipogenesis and visceral adipose tissue expansion. Thromb. Haemost. 116: 694–704.
Gelling, R. W., W. Yan, S. Al-Noori, A. Pardini, G. J. Morton, K. Ogimoto, M. W. Schwartz, and P. J. Dempsey (2008) Deficiency of TNFa converting enzyme (TACE/ADAM17) causes a lean, hypermetabolic phenotype in mice. Endocrinology. 149: 6053–6064.
Voros, G., E. Maquoi, D. Collen, and H. R. Lijnen (2003) Differential expression of plasminogen activator inhibitor-1, tumor necrosis factor-alpha, TNF-alpha converting enzyme and ADAMTS family members in murine fat territories. Biochim. Biophys. Acta. 1625: 36–42.
Vázquez, F., G. Hastings, M. A. Ortega, T. F. Lane, S. Oikemus, M. Lombardo, and M. L. Iruela-Arispe (1999) METH-1, a human ortholog of ADAMTS-1, and METH-2 are members of a new family of proteins with angio-inhibitory activity. J. Biol. Chem. 274: 23349–23357.
Luque, A., D. R. Carpizo, and M. L. Iruela-Arispe (2003) ADAMTS1/METH1 inhibits endothelial cell proliferation by direct binding and sequestration of VEGF165. J. Biol. Chem. 278: 23656–23665.
Chen, S. Z., L. F. Ning, X. Xu, W. Y. Jiang, C. Xing, W. P. Jia, X. L. Chen, Q. Q. Tang, and H. Y. Huang (2016) The miR-181d-regulated metalloproteinase Adamts1 enzymatically impairs adipogenesis via ECM remodeling. Cell Death Differ. 23: 1778–1791.
Kumar, S., S. Sharghi-Namini, N. Rao, and R. Ge (2012) ADAMTS5 functions as an anti-angiogenic and anti-tumorigenic protein independent of its proteoglycanase activity. Am. J. Pathol. 181: 1056–1068.
Lee, M. H., A. G. Goralczyk, R. Kriszt, X. M. Ang, C. Badowski, Y. Li, S. A. Summers, S. A. Toh, M. S. Yasin, A. Shabbir, A. Sheppard, and M. Raghunath (2016) ECM microenvironment unlocks brown adipogenic potential of adult human bone marrow-derived MSCs. Sci. Rep. 6: 21173.
Ruiz-Ojeda, F. J., J. Wang, T. Bäcker, M. Krueger, S. Zamani, S. Rosowski, T. Gruber, Y. Onogi, A. Feuchtinger, T. J. Schulz, R. Fässler, T. D. Müller, C. García-Cáceres, M. Meier, M. Blüher, and S. Ussar (2021) Active integrins regulate white adipose tissue insulin sensitivity and brown fat thermogenesis. Mol. Metab. 45: 101147.
Crawford, S. E., V. Stellmach, J. E. Murphy-Ullrich, S. M. Ribeiro, J. Lawler, R. O. Hynes, G. P. Boivin, and N. Bouck (1998) Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 93: 1159–1170.
Guilherme, A. and M. P. Czech (1998) Stimulation of IRS-1-associated phosphatidylinositol 3-kinase and Akt/protein kinase B but not glucose transport by beta1-integrin signaling in rat adipocytes. J. Biol. Chem. 273: 33119–33122.
Ueno, T. and K. Fujimori (2011) Novel suppression mechanism operating in early phase of adipogenesis by positive feedback loop for enhancement of cyclooxygenase-2 expression through prostaglandin F2α receptor mediated activation of MEK/ERK-CREB cascade. FEBS J. 278: 2901–2912.
Kim, H. B., W. H. Kim, K. L. Han, J. H. Park, J. Lee, J. Yeo, and M. H. Jung (2010) cAMP-response element binding protein (CREB) positively regulates mouse adiponectin gene expression in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 391: 634–639.
Shi, F. and S. Collins (2017) Second messenger signaling mechanisms of the brown adipocyte thermogenic program: an integrative perspective. Horm. Mol. Biol. Clin. Investig. 31: 20170062.
Acknowldgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2019R1A2C2002163).
The authors declare no conflict of interest.
Neither ethical approval nor informed consent was required for this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Choi, M.J., Mukherjee, S. & Yun, J.W. Loss of ADAMTS15 Promotes Browning in 3T3-L1 White Adipocytes via Activation of β3-adrenergic Receptor. Biotechnol Bioproc E 26, 188–200 (2021). https://doi.org/10.1007/s12257-021-0036-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-021-0036-y