Abstract
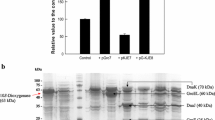
In this study, alcohol dehydrogenases (ADH) enzymes from Yarrowia lipolytica were investigated for the cloning, soluble expression, and biotransformation of 1-dodecanol to 1-dodecanal, which reaction was thermodynamically unfavorable. Sole expression of ADHs in Escherichia coli did not produce soluble form of cytosolic protein, in spite of the effort to solubilize ADH protein by optimizing IPTG concentration, temperature, and auto-induction medium. Eventually, the active form of soluble ADH proteins was successfully obtained through the co-expression of ADH with chaperone protein in pG-KJE8 vector. After analyzing the individual sets of optimization, it was determined that pET-28a(+)::adh coexpression with the pG-KJE8 molecular chaperone in LB medium with 0.1 mM IPTG, 4 mg/mL arabinose, and 2 ng/mL tetracycline achieved optimum expressions against all of the five ADH proteins. Finally, the whole cell biotransformation activity of ADH2 was determined in 1-dodecanol oxidation to 1-dodecanal, followed by further oxidation to 1-dodecanoic acid.
Similar content being viewed by others
References
Koesoema, A. A., D. M. Standley, T. Senda, and T. Matsuda (2020) Impact and relevance of alcohol dehydrogenase enantioselectivities on biotechnological applications. Appl. Microbiol. Biotechnol. 104: 2897–2909.
Dahlin, J., C. Holkenbrink, E. R. Marella, G. Wang, U. Liebal, C. Lieven, D. Weber, D. McCloskey, B. E. Ebert, M. J. Herrgard, L. M. Blank, I. Borodina, and H. L. Wang (2019) Multi-omics analysis of fatty alcohol production in engineered yeasts Saccharomyces cerevisiae and Yarrowia lipolytica. Front. Genet. 10: 747.
Gutheil, W. G., B. Holmquist, and B. L. Vallee (1992) Purification, characterization, and partial sequence of the glutathione-dependent formaldehyde dehydrogenase from Escherichia coli: a class III alcohol dehydrogenase. Biochemistry. 31: 475–481.
Danielsson, O. and H. Jornvall (1992) “Enzymogenesis”: classical liver alcohol dehydrogenase origin from the glutathione-dependent formaldehyde dehydrogenase line. Proc. Natl. Acad. Sci. USA. 89: 9247–9251.
Chang, Y. K., J. P. Chen, J. R. Sheu, P. J. Chen, C. H. Su, and S. Y. Chou (2006) Direct recovery of alcohol dehydrogenase from unclarified yeast cell homogenate by IDEBAC using an improved scheme for elution. Biochem. Eng J. 30: 1–10.
Kasprzak, K., F. Bischoff, M. Rauter, K. Becker, K. Baronian, R. Bode, F. Schauer, H. M. Vorbrodt, and G. Kunze (2016) Synthesis of 1-(S)-phenylethanol and ethyl (R)-4-chloro-3-hydroxybutanoate using recombinant Rhodococcus erythropolis alcohol dehydrogenase produced by two yeast species. Biochem. Eng. J. 106: 107–117.
Lee, J. S., W. J. Chi, S. K. Hong, J. W. Yang, and Y. K. Chang (2013) Bioethanol production by heterologous expression of Pdc and AdhII in Streptomyces lividans. Appl. Microbiol. Biotechnol. 97: 6089–6097.
Jones, I. G., V. Fairhurst, and H. M. Sealy-Lewis (2001) ADHII in Aspergillus nidulans is induced by carbon starvation stress. Fungal Genet Biol. 32: 33–43.
Ciriacy, M. (1976) Cis-dominant regulatory mutations affecting the formation of glucose-repressible alcohol dehydrogenase (ADHII) in Saccharomyces cerevisiae. Mol Gen Genet. 145: 327–333.
Choi, K. Y., D. G. Wernick, C. A. Tat, and J. C. Liao (2014) Consolidated conversion of protein waste into biofuels and ammonia using Bacillus subtilis. Metab. Eng. 23: 53–61.
Park, H. A. and K. Y. Choi (2020) α, ω-Oxyfunctionalization of C12 alkanes via whole-cell biocatalysis of CYP153A from Marinobacter aquaeolei and a new CYP from Nocardia farcinica IFM10152. Biochem. Eng. J. 156: 107524.
Yoo, H. W., J. Kim, M. D. Patil, B. G. Park, S. Y. Joo, H. Yun, and B. G. Kim (2019) Production of 12-hydroxy dodecanoic acid methyl ester using a signal peptide sequence-optimized transporter AlkL and a novel monooxygenase. Bioresour. Technol. 291: 121812.
Ahsan, M. M., H. Jeon, S. P. Nadarajan, T. Chung, H. W. Yoo, B. G. Kim, M. D. Patil, and H. Yun (2018) Biosynthesis of the Nylon 12 monomer, omega-aminododecanoic acid with novel CYP153A, AlkJ, and omega-TA enzymes. Biotechnol. J. 13: e1700562.
Xu, P., K. Qiao, W. S. Ahn, and G. Stephanopoulos (2016) Engineering Yarrowia lipolytica as a platform for synthesis of drop-in transportation fuels and oleochemicals. Proc. Natl. Acad. Sci. USA. 113: 10848–10853.
Lee, H., Y. E. C. Sugiharto, S. Lee, G. Park, C. Han, H. Jang, W. Jeon, H. Park, J. Ahn, K. Kang, and H. Lee (2017) Characterization of the newly isolated omega-oxidizing yeast Candida sorbophila DS02 and its potential applications in long-chain dicarboxylic acid production. Appl. Microbiol. Biotechnol. 101: 6333–6342.
Han, C., H. Kwon, G. Park, M. Jang, H. J. Lee, S. Seo, M. Kwon, W. Jeon, H. Lee, H. Lee, and J. Ahn (2020) Enhanced mating-type switching and sexual hybridization in heterothallic yeast Yarrowia lipolytica. FEMS Yeast Res. 20: foaa011.
Iwama, R., S. Kobayashi, C. Ishimaru, A. Ohta, H. Horiuchi, and R. Fukuda (2016) Functional roles and substrate specificities of twelve cytochromes P450 belonging to CYP52 family in n-alkane assimilating yeast Yarrowia lipolytica. Fungal Genet Biol. 91: 43–54.
Lee, H., C. Han, H. W. Lee, G. Park, W. Jeon, J. Ahn, and H. Lee (2018) Development of a promising microbial platform for the production of dicarboxylic acids from biorenewable resources. Biotechnol. Biofuels. 11: 310.
Park, H., I. Yang, M. Choi, K. S. Jang, J. C. Jung, and K. Y. Choi (2020) Engineering of melanin biopolymer by co-expression of MelC tyrosinase with CYP102G4 monooxygenase: Structural composition understanding by 15 tesla FT-ICR MS analysis. Biochem. Eng. J. 157: 107530.
Iwama, R., S. Kobayashi, A. Ohta, H. Horiuchi, and R. Fukuda (2015) Alcohol dehydrogenases and an alcohol oxidase involved in the assimilation of exogenous fatty alcohols in Yarrowia lipolytica. FEMS Yeast Res. 15: fov014.
de Smidt, O., J. C. du Preez, and J. Albertyn (2008) The alcohol dehydrogenases of Saccharomyces cerevisiae: a comprehensive review. FEMS Yeast Res. 8: 967–978.
Kusano, M., Y. Sakai, N. Kato, H. Yoshimoto, H. Sone, and Y. Tamai (1998) Hemiacetal dehydrogenation activity of alcohol dehydrogenases in Saccharomyces cerevisiae. Biosci. Biotechnol. Biochem. 62: 1956–1961.
Wales, M. R. and C. A. Fewson (1994) NADP-dependent alcohol dehydrogenases in bacteria and yeast: purification and partial characterization of the enzymes from Acinetobacter calcoaceticus and Saccharomyces cerevisiae. Microbiology (Reading). 140: 173–183.
Johnson, J., Y. H. Yang, D. G. Lee, J. J. Yoon, and K. Y. Choi (2018) Expression, purification and characterization of halophilic protease Pph_Pro1 cloned from Pseudoalteromonas phenolica. Protein Expr. Purif. 152: 46–55.
Sudheer, P. D. V. N., J. Yun, S. Chauhan, T. J. Kang, and K. Y. Choi (2017) Screening, expression, and characterization of Baeyer-Villiger monooxygenases for the production of 9-(nonanoyloxy)nonanoic acid from oleic acid. Biotechnol. Bioprocess Eng. 22: 717–724.
Sudheer, P. D. V. N., D. Seo, E. J. Kim, S. Chauhan, J. R. Chunawala, and K. Y. Choi (2018) Production of (z)-11-(heptanoyloxy)undec-9-enoic acid from ricinoleic acid by utilizing crude glycerol as sole carbon source in engineered Escherichia coli expressing BVMO-ADH-FadL. Enzyme Microb. Technol. 119: 45–51.
Nguyen, G. T., Y. G. Kim, J. W. Ahn, and J. H. Chang (2020) Structural basis for broad substrate selectivity of alcohol dehydrogenase YjgB from Escherichia coli. Molecules. 25: 2404.
Liu, H. L., Y. Ho, and C. M. Hsu (2003) The effect of metal ions on the binding of ethanol to human alcohol dehydrogenase beta2beta2. J. Biomed. Sci. 10: 302–312.
De Bolle, X., C. Vinals, J. Fastrez, and E. Feytmans (1997) Bivalent cations stabilize yeast alcohol dehydrogenase I. Biochem. J. 323: 409–413.
Acknowldgements
This work was supported by the Industrial Strategic Technology Development program (No. 20002734).
Author information
Authors and Affiliations
Corresponding author
Additional information
Ethical Statements
The authors declare no conflict of interest. Neither ethical approval nor informed consent was required for this study.
Authorship Contribution Statements
Ji-Hwan Jang: Validation, Visualization, Writing - review & editing.
Kwon-Young Choi: Writing - original draft, Writing - review & editing.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jang, JH., Choi, KY. Whole Cell Biotransformation of 1-dodecanol by Escherichia coli by Soluble Expression of ADH Enzyme from Yarrowia lipolytica. Biotechnol Bioproc E 26, 247–255 (2021). https://doi.org/10.1007/s12257-020-0176-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-020-0176-5