Abstract
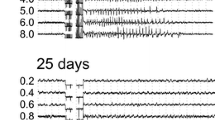
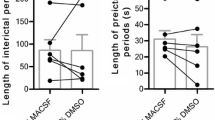
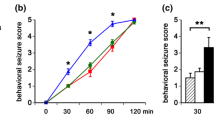
Children are more likely to develop epileptic seizures (ictal discharges lasting tens of seconds) than adults. A higher predisposition of the juvenile brain to the generation of epileptiform activity is thought to be due to a prevalence of inhibition over excitation at the early stage of brain development. However, the molecular and physiological mechanisms underlying these age-related differences are yet to be clarified. We compared the ictal activity induced by a convulsant 4-aminopyridine (4-AP) in the horizontal slices of the entorhinal cortex and hippocampus of 3- and 8-week-old Wistar rats. In 3-week-old rats, the ictal discharge was always preceded by a detectable preictal activity, as manifested in one or several 3–4-s GABA-glutamate events, whereas in 8-week-old rats, such events were typically absent or very rare (no more than one occasional short event). The ictal activity resistance to external exposures was also age-dependent. In 8-week-old rats, by contrast to 3-week-old animals, ictal discharge generation in the entorhinal cortex was blocked completely and replaced by 0.2–0.3 Hz interictal activity (simultaneous 1–3-s burst discharges) by a partial blockade of KCC2 cotransporter or Na+–K+-pump, as well as by low-frequency electric stimulation. Thus, our data indicate that ictal discharges in the immature (3-week-old) brain are more resistant to external exposures than in the brain of adult rats. Interictal and ictal epileptiform activities are antagonistic in 8-week-old animals. In contrast, the appearance of interictal activity interrupts the generation of ictal discharges completely. It can therefore be considered as one of the putative antiepileptic mechanisms in the mature rat brain.






Similar content being viewed by others
REFERENCES
Cowan, L.D., The epidemiology of the epilepsies in children, Ment. Retard. Dev. Disabil. Res. Rev., 2002, vol. 8, pp. 171–181. https://doi.org/10.1002/mrdd.10035
Studenikin, V.M., Shelkovsky, V.I., and Balkanskaya, S.V., Febrile seizures, Prakt. Ped., 2007, vol. 1, pp. 8–10.
Hauser, W.A., Incidence and prevalence, Epilepsy: A Comprehensive Textbook, Engel, J. Jr. and Pedley, T.A., Eds., Lippincott Raven Publishers, Philadelphia, pp. 47–58, 1997. https://doi.org/10.1007/978-88-470-2903-3_81
Plotkin, M.D., Snyder, E.Y., Hebert, S.C., and Delpire, E., Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains: a possible mechanism underlying GABA’s excitatory role in immature brain, J. Neurobiol., 1997, vol. 33(6), pp. 781–795. https://doi.org/10.1002/(sici)1097-4695(19971120)33:6<781::aid-neu6>3.0.co;2-5
Wang, C., Shimizu-Okabe, C., Watanabe, K., Okabe, A., Matsuzaki, H., Ogawa, T., Mori, N., Fukuda, A., and Sato, K., Developmental changes in KCC1, KCC2, and NKCC1 mRNA expressions in the rat brain, Dev. Brain Res., 2002, vol. 139(1), pp. 59–66. https://doi.org/10.1016/s0165-3806(02)00536-9
Dzhala, V.I., Talos, D.M., Sdrulla, D.A., Brumback, A.C., Mathews, G.C., Benke, T.A., Delpire, E., Jensen, F.E., and Staley, K.J., NKCC1 transporter facilitates seizures in the developing brain, Nat. Med., 2005, vol. 11(11), pp. 1205–1213. https://doi.org/10.1038/nm1301
Khazipov, R., Khalilov, I., Tyzio, R., Morozova, E., Ben-Ari, Y., and Holmes, G.L., Developmental changes in GABAergic actions and seizure susceptibility in the rat hippocampus, Eur. J. Neurosci., 2004, vol. 19(3), pp. 590–600. https://doi.org/10.1111/j.0953-816x.2003.03152.x
Watanabe, M. and Fukuda, A., Development and regulation of chloride homeostasis in the central nervous system, Front. Cell Neurosci., 2015, vol. 9, p. 371. https://doi.org/10.3389/fncel.2015.00371
Lei, S. and McBain, C.J., GABAB receptor modulation of excitatory and inhibitory synaptic transmission onto rat CA3 hippocampal interneurons, J. Physiol., 2003, vol. 15(2), 546, pp. 439–453. https://doi.org/10.1113/jphysiol.2002.034017
Nurse, S. and Lacaille, J.C., Late maturation of GABA(B) synaptic transmission in area CA1 of the rat hippocampus, Neuropharmacol., 1999, vol. 38(11), 1733e42. https://doi.org/10.1016/s0028-3908(99)00122-7
Scanziani, M., GABA spillover activates postsynaptic GABA(B) receptors to control rhythmic hippocampal activity, Neuron, 2000, vol. 25(3), pp. 673–681. https://doi.org/10.1016/S0896-6273(00)81069-7
Sundaram, S.M., Safina, D., Ehrkamp, A., Faissner, A., Heumann, R., and Dietzel, I.D., Differential expression patterns of sodium potassium ATPase alpha and beta subunit isoforms in mouse brain during postnatal development, Neurochem. Int., 2019, vol. 128, pp. 163–174. https://doi.org/10.1016/j.neuint.2019.04.009
Banerjee, B. and Chaudhury, S., Thyroidal regulation of different isoforms of NaKATPase in the primary cultures of neurons derived from fetal rat brain, Life Sci., 2002, vol. 71(14), pp. 1643–1654. https://doi.org/10.1016/s0024-3205(02)01856-8
Amakhin, D.V., Ergina, J.L., Chizhov, A.V., and Zaitsev, A.V., Synaptic conductances during interictal discharges in pyramidal neurons of rat entorhinal cortex, Front. Cell Neurosci., 2016, vol. 10, p. 233. eCollection. https://doi.org/10.3389/fncel.2016.00233
Yao, J.A. and Tseng, G.N., Modulation of 4-AP block of a mammalian A-type K channel clone by channel gating and membrane voltage, Biophys. J., 1994, vol. 67(1), pp. 130–142. https://doi.org/10.1016/S0006-3495(94)80462-X
Choquet, D. and Korn, H., Mechanism of 4-aminopyridine action on voltage-gated potassium channels in lymphocytes, J. Gen. Physiol., 1992, vol. 99(2), pp. 217–240. https://doi.org/10.1085/jgp.99.2.217
Octeau, J.C., Faas, G., Mody, I., and Khakh, B.S., Making, testing, and using potassium ion selective microelectrodes in tissue slices of adult brain, J. Vis. Exp., 2018, (135), 57511. https://doi.org/10.3791/57511
Malyshev, A.Y., Roshchin, M.V., Smirnova, G.R., Dolgikh, D.A., Balaban, P.M., and Ostrovsky, M.A., Chloride conducting light activated channel GtACR2 can produce both cessation of firing and generation of action potentials in cortical neurons in response to light, Neurosci. Let., 2017, vol. 640, pp. 76–80. https://doi.org/10.1016/j.neulet.2017.01.026
Yu, Y., Shu, Y., and McCormick, D.A., Cortical action potential backpropagation explains spike threshold variability and rapid-onset kinetics, J. Neurosci., 2008, vol. 28(29), pp. 7260–7272. https://doi.org/10.1523/JNEUROSCI.1613-08.2008
Tai, Y., Gallo, N.B., Wang, M., Yu, J.R., and Van Aelst, L., Axo-axonic innervation of neocortical pyramidal neurons by GABAergic chandelier cells requires ankyrin G-associated L1CAM, Neuron, 2019, vol. 102(2), pp. 358–372, e9. https://doi.org/10.1016/j.neuron.2019.02.009
Ben-Ari, Y., Gaiarsa, J.L., Tyzio, R., and Khazipov, R., GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations, Physiol. Rev., 2007, vol. 87(4), pp. 1215–1284. https://doi.org/10.1152/physrev.00017.2006
Huberfeld, G., Wittner, L., Clemenceau, S., Baulac, M., Kaila, K., Miles, R., and Rivera, C., Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy, J. Neurosci., 2007, vol. 27, pp. 9866–9873. https://doi.org/10.1523/JNEUROSCI.2761-07.2007
Kaila, K., Ruusuvuori, E., Seja, P., Voipio, J., and Puskarjov, M., GABA actions and ionic plasticity in epilepsy, Curr. Opin. Neurobiol., 2014, vol. 26, pp. 34–41. https://doi.org/10.1016/j.conb.2013.11.004
Chizhov, A.V., Zefirov, A.V., Amakhin, D.V., Smirnova, E.Y., and Zaitsev, A.V., Minimal model of interictal and ictal discharges “Epileptor-2”, PLoS Comput Biol., 2018, vol. 14(5), e1006186. https://doi.org/10.1371/journal.pcbi.1006186
Smirnova, E.Y., Chizhov, A.V., and Zaitsev, A.V., Presynaptic GABAB receptors underlie the antiepileptic effect of low-frequency electrical stimulation in the 4-aminopyridine model of epilepsy in brain slices of young rats, Brain Stimul., 2020, vol. 13(5), pp. 1387–1395. https://doi.org/10.1016/j.brs.2020.07.013
Swartzwelder, H.S., Lewis, D.V., Anderson, W.W., and Wilson, W.A., Seizure-like events in brain slices: suppression by interictal activity, Brain Res., 1987, vol. 410(2), pp. 362–366. https://doi.org/10.1016/0006-8993(87)90339-8
Barbarosie, M. and Avoli, M., CA3-driven hippocampal-entorhinal loop controls rather than sustains in vitro limbic seizures, J. Neurosci., 1997, vol. 17(23), pp. 9308–9314. https://doi.org/10.1523/JNEUROSCI.17-23-09308.1997
Berdichevsky, Y., Dzhala, V., Mail, M., and Staley, K.J., Interictal spikes, seizures and ictal cell death are not necessary for post-traumatic epileptogenesis in vitro, Neurobiol. Dis., 45(2):774-785. 2012. https://doi.org/10.1016/j.nbd.2011.11.001
Jabès, A., Lavenex, P.B., Amaral, D.G., and Lavenexs, P., Postnatal development of the hippocampal formation: a stereological study in macaque monkeys, J. Comp. Neurol., 2011, vol. 519(6), pp. 1051–1070. https://doi.org/10.1002/cne.22549
Magloire, V., Cornford, J., Lieb, A., Kullmann, D.M., and Pavlov, I., KCC2 overexpression prevents the paradoxical seizure-promoting action of somatic inhibition, Nat. Commun., 2019, vol. 10(1), p. 1225. https://doi.org/10.1038/s41467-019-08933-4
Puskarjov, M., Ahmad, F., Kaila, K., and Blaesse, P., Activity-dependent cleavage of the K–Cl cotransporter KCC2 mediated by calcium-activated protease calpain, J. Neurosci., 2019, vol. 32(33), pp. 11356–11364. https://doi.org/10.1523/JNEUROSCI.6265-11.2012
Kahle, K.T., Merner, N.D., Friedel, P., Silayeva, L., Liang, B., Khanna, A., Shang, Y., Lachance-Touchette, P., Bourassa, C., Levert, A., Dion, P.A., Walcott, B., Spiegelman, D., Dionne-Laporte, A., Hodgkinson, A., Awadalla, P., Nikbakht, H., Majewski, J., Cossette, P., Deeb, T.Z., Moss, S.J., Medina, I., and Rouleau, G.A., Genetically encoded impairment of neuronal KCC2 cotransporter function in human idiopathic generalized epilepsy, EMBO Rep., 2014, vol. 15(7), pp. 766–774. https://doi.org/10.1523/JNEUROSCI.6265-11.2012
Ellender, T.J., Raimondo, J.V., Irkle Lamsa, K.P., and Akerman, C.J., Excitatory effects of parvalbumin-expressing interneurons maintain hippocampal epileptiform activity via synchronous afterdischarges, J. Neurosci., 2014, vol. 34, pp. 15208–15222. https://doi.org/10.1523/JNEUROSCI.1747-14.2014
Nardou, R., Yamamoto, S., Chazal, G., Bhar, A., Ferrand, N., Dulac, O., Ben-Ari, Y., and Khalilov, I., Neuronal chloride accumulation and excitatory GABA underlie aggravation of neonatal epileptiform activities by phenobarbital, Brain, 2011, vol. 134, pp. 987–1002. https://doi.org/10.1093/brain/awr041
Toprani, S. and Durand, D.M., Long-lasting hyperpolarization underlies seizure reduction by low frequency deep brain electrical stimulation, J. Physiol., 2013, vol. 591(22), 5765e90. https://doi.org/10.1113/jphysiol.2013.253757
Kano, T., Inaba, Y., D’Antuono, M., Biagini, G., Levesque, M., and Avoli, M., Blockade of in vitro ictogenesis by low-frequency stimulation coincides with increased epileptiform response latency, J. Neurophysiol., 2015, vol. 114(1), 21e8. https://doi.org/10.1152/jn.00248.2015
Funding
The reported study was funded by the Russian Foundation for Basic Research, project number 19-315-60016.
Author information
Authors and Affiliations
Contributions
E.Yu. Smirnova: experimental design, data collection and processing, writing and editing the manuscript. D.S. Sinyak: data collection on the blockade of the Na+–K+-pump and KCC2 cotransporter in brain slices of 3-week-old rats. A.V. Chizhov and A.V. Zaitsev: data discussion, editing the manuscript.
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
The experimental protocol met the requirements of the Ethics Committee at the Sechenov Institute of Evolutionary Physiology and Biochemistry (Russian Academy of Sciences) based on international recommendations of the European Communities Council Directive of 1986 (86/609/EEC).
This study did not involve human subjects as research objects.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Additional information
Russian Text © The Author(s), 2021, published in Zhurnal Evolyutsionnoi Biokhimii i Fiziologii, 2021, Vol. 57, No. 2, pp. 144–153https://doi.org/10.31857/S0044452921020078.
Translated by A. Polyanovsky
Rights and permissions
About this article
Cite this article
Smirnova, E.Y., Sinyak, D.S., Chizhov, A.V. et al. Age-Dependent Generation of Epileptiform Activity in the 4-Aminopyridine Model with Slices of the Rat Entorhinal Cortex. J Evol Biochem Phys 57, 230–240 (2021). https://doi.org/10.1134/S0022093021020058
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093021020058