Abstract
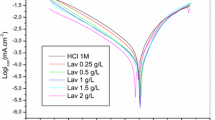
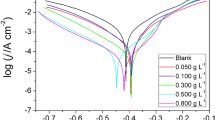
The obtained results allowed to reveal the inhibition properties of the essential oils of Foeniculum vulgare and to identify the major molecule—trans-anethole that enters into their composition. In this work, an isolated molecule of trans-anethole was tested in order to confirm its responsibility for a high inhibition efficiency and to study its adsorption mechanism. The inhibition efficiency of this natural molecule was evaluated by using such techniques as electrochemical polarization, impedance spectroscopy, and weight loss measurements. A protective layer formed on the mild steel surface was analyzed by scanning electron microscopy. This study showed a maximal inhibitory efficiency at 1.0 × 10–3 M. The essential oils of Foeniculum vulgare were found to be a mixed type inhibitor that adsorbs on the metal surface by physisorption and is resistant to high temperatures. Finally, the results obtained allowed not only to determine the molecule responsible for a good anticorrosive activity of three oils extracted from three parts of Foeniculum vulgare, but also to obtain a new strong ecological inhibitor.













Similar content being viewed by others
REFERENCES
Loto, R.T., Olukeye, T., and Okorie, E., Synergistic combination effect of clove essential oil extract with basil and atlas cedar oil on the corrosion inhibition of low carbon steel, S. Afr. J. Chem. Eng., 2019, vol. 30, p. 28.
Oyekunle, D.T., Oguntade, T.I., Ita, C.S., Ojo, T., et al., Corrosion inhibition of mild steel using binary mixture of sesame and castor oil in brine solution, Mater. Today Commun., 2019, vol. 21, p. 100691.
Ostovari, A., Hoseinieh, S.M., Peikari, M., Shadizadeh, S.R., and Hashemi, S.J., Corrosion inhibition of mild steel in 1 M HCl solution by henna extract: a comparative study of the inhibition by henna and its constituents (Lawsone, gallic acid, α-D-glucose and tannic acid), Corros. Sci., 2009, vol. 51, pp. 1935–1949.
Abiola, O.K., Otaigbe, J.O.E., and Kio, O.J., Gossipium hirsutum L. extracts as green corrosion inhibitor for aluminum in NaOH solution, Corros. Sci., 2009, vol. 51, p. 1879.
Okafor, P.C., Osabor, V.I., and Ebenso, E.E., Eco-friendly corrosion inhibitors: inhibitive action of ethanol extracts of Garcinia kola for the corrosion of mild steel in H2SO4 solutions, Pigm. Res. Technol., 2007, vol. 35, p. 299.
Okafor, P.C. and Ebenso, E.E., Furan and phenyl substituted triazolothiadiazine derivatives as copper corrosion inhibitors: electrochemical and DFT studies, Pigm. Res. Technol., 2007, vol. 36, p. 134.
Ogan, A.U., The basic constituents of the leaves of Carica papaya, Phytochem. Rep., 1971, vol. 99, p. 441.
Okafor, P.C., Ikpi, M.E., Uwah, I.E., Ebenso, E.E., et al., Inhibitory action of Phyllanthus amarus extracts on the corrosion of mild steel in acidic media, Corros. Sci., 2008, vol. 50, p. 2310.
Srivastava, K. and Srivastava, P., Studies on plant material as corrosion inhibitors, Br. Corros. J., 1981, vol. 16, p. 221.
Rather, M.A., Dar Bilal, A., Sofi Shahnawaz, N., Bhat, B.A., et al., Foeniculum vulgare: A comprehensive review of its traditional use, phytochemistry, pharmacology, and safety, Arab. J. Chem., 2016, vol. 9, suppl. 2, p. S1574.
Akhtar, I., Javad, S., Ansari, M., Ghaffar, N., et al., Process optimization for microwave assisted extraction of Foeniculum vulgare Mill using response surface methodology, J. King Saud Univ., Sci., 2019, vol. 32, no. 2, p. 1451. https://doi.org/10.1016/j.jksus.2019.11.041
El-Hajjaji, F., Messali, M., Aljuhani, A., Aouad, M.R., et al., Pyridazinium-based ionic liquids as novel and green corrosion inhibitors of carbon steel in acid medium: electrochemical and molecular dynamics simulation studies, J. Mol. Liq., 2018, vol. 249, p. 997.
Bouoidina, A., Chaouch, M. Abdellaoui, A.F., Lahkimi, A., et al., Essential oil of Foeniculum vulgare: Antioxidant and corrosion inhibitor on mild steel immersed in hydrochloric medium, Anti-Corros. Methods Mater., 2017, vol. 64, no. 5, p. 563.
Bouoidina, A., El-Hajjaji, F., Abdellaoui, A., Rais, Z., et al., Theoretical and experimental study of the corrosion inhibition of mild steel in acid medium using some surfactants of the essential oil of Foeniculum vulgare bulb, J. Mater. Environ. Sci., 2017, vol. 8, no. 4, p. 1328.
Dagdag, O., El Harfi, A., El Gouri, M., Safi, Z., et al., Anticorrosive properties of hexa (3-methoxy propan-1,2-diol) cyclotri-phosphazene compound for carbon steel in 3% NaCl medium: Gravimetric, electrochemical, DFT and Monte Carlo simulation studies, Heliyon, 2019, vol. 5, no. 3, p. e01340.
Bouoidina, A., El-Hajjaji, F., Drissi, M., Taleb, M., Hammouti, B., Ill-Min, C., Jodeh, S., and Lgaz, H., Towards a deeper understanding of the anticorrosive properties of hydrazine derivatives in acid medium: Experimental, DFT and MD simulation assessment, Metall. Mater. Trans. A, 2018, vol. 49, p. 5180.
El-Hajjaji, F., Messali, M., Martinez de Yuso, M.V., Rodriguez-Castellon, E., et al., Effect of 1-(3-phenoxypropyl) pyridazin-1-ium bromide on steel corrosion inhibition in acidic medium, J. Colloid Interface Sci., 2019, vol. 541, p. 418.
Bouoidina, A., El-Hajjaji, F., Emran, K., Belghiti, M.E, et al., Towards understanding the anticorrosive mechanism of novel surfactant based on Mentha pulegium oil as eco-friendly bio-source of mild steel in acid medium: a combined DFT and molecular dynamics investigation, Chem. Res. Chin. Univ., 2019, vol. 35, p. 6.
Gualdron, A.F., Becerra, E.N., Pena, Dario, Y., Gutierrez, J.C., et al., Inhibitory effect of Eucalyptus and Lippia alba essential oils on the corrosion of mild steel in hydrochloric acid, J. Mater. Environ. Sci., 2013, vol. 4, no. 1, p. 143.
Bammou, L., Salghi, R., Zarrouk, A., Zarrok, H., et al., Inhibition effect of natural Junipers extract towards steel corrosion in HCl solution, Int. J. Electrochem. Sci., 2012, vol. 7, p. 8974.
Amar, H., Tounsi, A., Makayssi, A., Derja, A., et al., Corrosion inhibition of Armco iron by 2-mercaptobenzimidazole in sodium chloride 3% media, Corros. Sci., 2007, vol. 49, p. 2936.
Jafari, H., Akbarzade, K., and Danaee, I., Corrosion inhibition of carbon steel immersed in a 1 M HCl solution using benzothiazole derivatives, Arab. J. Chem., 2019, vol. 12, no. 7, p. 1387.
Karthik, G. and Sundaravadivelu, M., Studies on the inhibition of mild steel corrosion in hydrochloric acid solution by atenolol drug, Egypt. J. Petrol., 2016, vol. 25, no. 2, p. 183.
Ouakki, M., Galai, M., Rbaa, M., Abousalem, A.S., et al., Quantum chemical and experimental evaluation of the inhibitory action of two imidazole derivatives on mild steel corrosion in sulphuric acid medium, Heliyon, 2019, vol. 5, p. e02759.
Sigircik, G., Tuken, T., and Erbil, M., Assessment of the inhibition efficiency of 3,4-diaminobenzonitrile against the corrosion of steel, Corros. Sci., 2016, vol. 102, p. 437.
Ribeiro, D.V., Souza, C.A.C., and Abrantes, J.C.C., Use of Electrochemical Impedance Spectroscopy (EIS) to monitoring the corrosion of reinforced concrete, Rev. IBRACON Estruturas Mater., 2015, vol. 8, no. 4, p. 529.
Sherif El-S.M., Erasmus, R.M., and Comins, J.D., Effects of 3-amino-1,2,4-triazole on the inhibition of copper corrosion in acidic chloride solutions, J. Colloid Interface Sci., 2007, vol. 311, p. 144.
Li, L.L., Mo, S., Qun, L.H., Jun, F.Y., et al., Relationship between inhibition performance of melamine derivatives and molecular structure for mild steel in acid solution, Corros. Sci., 2017, vol. 124, p. 167, https://doi.org/10.1016/j.corsci.2017.05.020
Garai, S., Jaisankar, P., Singh, J.K., and Elango, A., A comprehensive study on crude methanolic extract of Artemisia pallens (Asteraceae) and its active component as effective corrosion inhibitors of mild steel in acid solution, Corros. Sci., 2012, vol. 60, p. 193.
Langmuir, I., The constitution and fundamental properties of solids and liquids. II. Liquids, J. Am. Chem. Soc., 1917, vol. 39, p. 1848.
Rahmani, H., Alaoui, K.I., M. Emran, K., El Hallaoui, A., Taleb, M., El Hajji, S., Labriti, B., Ech-chihbi, E., Hammouti, B., and El-Hajjaji, F., Experimental and DFT investigation on the corrosion inhibition of mild steel by 1,2,3-triazole regioisomers in 1 M hydrochloric acid solution, Int. J. Electrochem. Sci., 2019, vol. 14, p. 985.
El Azzouzi, M., Aouniti, A., Tighadouin, S., Elmsellem, H., et al., Some hydrazine derivatives as corrosion inhibitors for mild steel in 1.0 M HCl: Weight loss, electrochemichal, SEM and theoretical studies, J. Mol. Liq., 2016, vol. 221, p. 633. https://doi.org/10.1016/j.molliq.2016.06.007
Kowsari, E., Payami, M., Amini, R., Ramezanzadeh, B., et al., Task-specific ionic liquid as a new green inhibitor of mild steel corrosion, Appl. Surf. Sci., 2014, vol. 289, p. 478.
Obot, I.B., Umoren, S.A., Gasem, Z.M., Suleiman, R., et al., Theoretical prediction and electrochemical evaluation of vinylimidazole and allylimidazole as corrosion inhibitors for mild steel in 1 M HCl, J. Ind. Eng. Chem., 2015, vol. 21, p. e1328.
El Hajjaji, F., Abrigach, F., Hamed, O., Rasem, H.A., et al., Corrosion resistance of mild steel coated with organic material containing pyrazol moiety, Coatings, 2018, vol. 8, p. 330.
Fragoza-Mar, L., Olivares-Xometl, O., Domínguez-Aguilar, M.A., Flores, E.A., et al., Corrosion inhibitor activity of 1,3-diketone malonates for mild steel in aqueous hydrochloric acid solution, Corros. Sci., 2012, vol. 61, p. 171.
Bouhrira, K., Ouahiba, F., and Zerouali, D., The inhibitive effect of 2-phenyl-3-nitroso-imidazo [1,2-a]pyridine on the corrosion of steel in 0.5 M HCl acid solution, Eur. J. Chem., 2010, vol. 7, p. 35.
Ech-chihbi, E., Belghiti, M.E., Salim, R., Oudda, H., et al., Experimental and computational studies on the inhibition performance of the organic compound “2‑phenylimidazo [1,2-a]pyrimidine-3-carbaldehyde” against the corrosion of carbon steel in 1.0 M HCl solution, Surf. Interfaces, 2017, vol. 9, p. 206.
Ouakki, M., Rbaa, M., Galai, M., Lakhrissi, B., Rifi, E.H., and Cherkaoui, M., Experimental and quantum chemical investigation of imidazole derivatives as corrosion inhibitors on mild steel in 1.0 M hydrochloric acid, Journal of Bio- and Tribo-Corrosion., 2018, vol. 4, p. 35. https://doi.org/10.1007/s40735-018-0151-2
Soltani, N., Behpour, M., Ghoreishi, S.M., and Naeimi, H., Corrosion inhibition of mild steel in hydrochloric acid solution by some double Schiff bases, Corros. Sci., 2010, vol. 52, pp. 1351–1361.
Gutierrez, E., Rodriguez, J., Cruz-Borbolla, J., Alvarado-Rodriguez, J., and Thangarasu, P., Development of a predictive model for corrosion inhibition of carbon steel by imidazole and benzimidazole derivatives, Corros. Sci., 2016, vol. 108, p. 23. https://doi.org/10.1016/j.corsci.2016.02.036
Kumar, D., Jain, V., and Rai, B., Unravelling the mechanisms of corrosion inhibition of iron by henna extract: A density functional theory study, Corros. Sci., 2018, vol. 142, p. 102. https://doi.org/10.1016/j.corsci.2018.07.011
Ech-chihbi, E., Nahle, A., Salim, R., Oudda, H., et al., An investigation into quantum chemistry and experimental evaluation of imidazopyridine derivatives as corrosion inhibitors for C-steel in acidic media, Journal of Bio- and Tribo-Corrosion, 2019, vol. 5, p. 24.
Lukovits, I., Kalman, E., and Zucchi, F., Corrosion inhibitors-correlation between electronic structure and efficiency, Corrosion, 2001, vol. 57, p. 3.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Bouoidina, A., El-Hajjaji, F., Ech-chihbi, E. et al. Towards Discovery of Ecological Inhibitor for Corrosion of Mild Steel in HCl Medium: trans-Anethole—the Molecule Responsible for Inhibitory Efficiency of Essential Oils Extracted from Leaves, Seeds, and Bulbs of Foeniculum vulgare . Surf. Engin. Appl.Electrochem. 57, 255–267 (2021). https://doi.org/10.3103/S1068375521020022
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068375521020022