Effects of Dietary Energy Level on Performance, Plasma Parameters, and Central AMPK Levels in Stressed Broilers
- 1Department of Animal Science, Shandong Agricultural University, Taian, China
- 2Precision Livestock and Nutrition Unit, Gembloux Agro-Bio Tech, University of Liège, Gembloux, Belgium
This study aimed to characterize the effects of diets with different energy levels on the growth performance, plasma parameters, and central AMPK signaling pathway in broilers under dexamethasone (DEX)-induced stress. A total of 216 1-day-old male broiler chickens were allocated to groups fed with high (HED), National Research Council-recommended (control), or low (LED) energy diets. At 10 days old, chickens were treated with or without dexamethasone (DEX, 2 mg/kg body weight) for 3 consecutive days. HED increased broiler average daily gain (ADG) at 10 days old, compared with the LED (P < 0.05), while average daily feed intake (ADFI) and feed conversion rate (FCR) decreased as the dietary energy level increased (P < 0.05). Chickens fed a HED had higher total protein (TP) content, albumin (ALB), glucose (GLU), total cholesterol (TCHO), high-density lipoprotein (HDL) cholesterol, and low-density lipoprotein (LDL) cholesterol, compared with the control group (P < 0.05). At 13 days old, DEX decreased ADG and increased FCR in broilers fed with different energy diets (P < 0.05). The DEX-HED group had a higher ADFI than non-DEX treated HED group chickens. In addition, TP, ALB, triglycerides (TG), TCHO, HDL, and LDL content levels in the DEX group were higher than those in the control group (P < 0.05). The uric acid (UA) content of the LED group was higher than that of the HED group (P < 0.05). Further, gene expression levels of liver kinase B1, AMP-activated protein kinase α1, neuropeptide Y, and GC receptor in the hypothalamus were increased in chickens treated with DEX (P < 0.05). There was a trend toward interaction between plasma TCHO and hypothalamic LKB1 expression (0.05 < P < 0.1). In conclusion, this study suggests that HED improves growth performance, plasma glucose and total cholesterol at 10 days old broilers, but had no significant effect on performance, plasma parameters, and central AMPK in stressed broilers.
Introduction
Broiler chickens in intensive poultry production systems are challenged by various stress factors (1), including high temperature, high stock density, and diseases. Those factors may impair productive performance and survival, thereby resulting in financial losses for farmers (2, 3). Under stressful conditions, the total energy budget of an animal must be divided optimally among different physiological functions, such as thermoregulation, growth, and reproduction (4). Stress activates the hypothalamic-pituitary-adrenal (HPA) axis, promoting the release of glucocorticoids (GCs) to induce a physiological response to the stressor (5). GC, which are end products of the HPA axis, regulate the basal and stress-related homeostasis (6, 7) and stimulate the appetite in the central nervous system (CNS), facilitating nutrient uptake (8).
Metabolizable energy (ME), a macronutrient composition of diet, is a pivotal factor that influences the feed efficiency, growth performance, and carcass composition of poultry (9, 10). Many recent studies have addressed human and animal food preferences under stress conditions (11–13). Stress increases the preference for high-fat foods in rodents (14, 15) and in humans (16). Further, some studies have found that high-fat diets affect stress response modulation by reducing the autonomic and HPA axis responses to repeated stressors in rodents (14, 17–19). Under stress, chickens may be able to detect metabolic changes (20) and prefer to consume a high-energy diet (1).
The hypothalamus can integrate signals from the brain, peripheral circulation, and gastrointestinal tract to regulate feeding and energy balance (21, 22). The hypothalamus, particularly the arcuate nucleus (ARC), contains two neuron populations that control feed intake, energy balance, and glucose (GLU) homeostasis, and agouti-related peptide/neuropeptide Y (AgRP/NPY)-releasing neurons are orexigenic in the ARC of avian species and mammals (23–25). AMP-activated protein kinase (AMPK) is involved in the regulation of cellular energy homeostasis, which is activated by an increased AMP to ATP ratio in mammals (26). In addition, the liver kinase B1 (LKB1)/AMPK pathway has a similar function in mammals and chickens (27). GC, via the hypothalamic AMPK pathway, induce a preference for food rich in fat (28).
To date, the effects of different energy diets on stress and its underlying mechanisms in broiler chickens remain unknown. In this study, we conducted experiments using different energy diets, and administered subcutaneous injections of dexamethasone (DEX), a synthetic glucocorticoid, to mimic stress (29–31). The aim of this study was to investigate the effects of different energy level diets on the performance, plasma composition, and central AMPK signaling in stressed broiler chickens.
Materials and Methods
Experimental Animals, Design, and Management
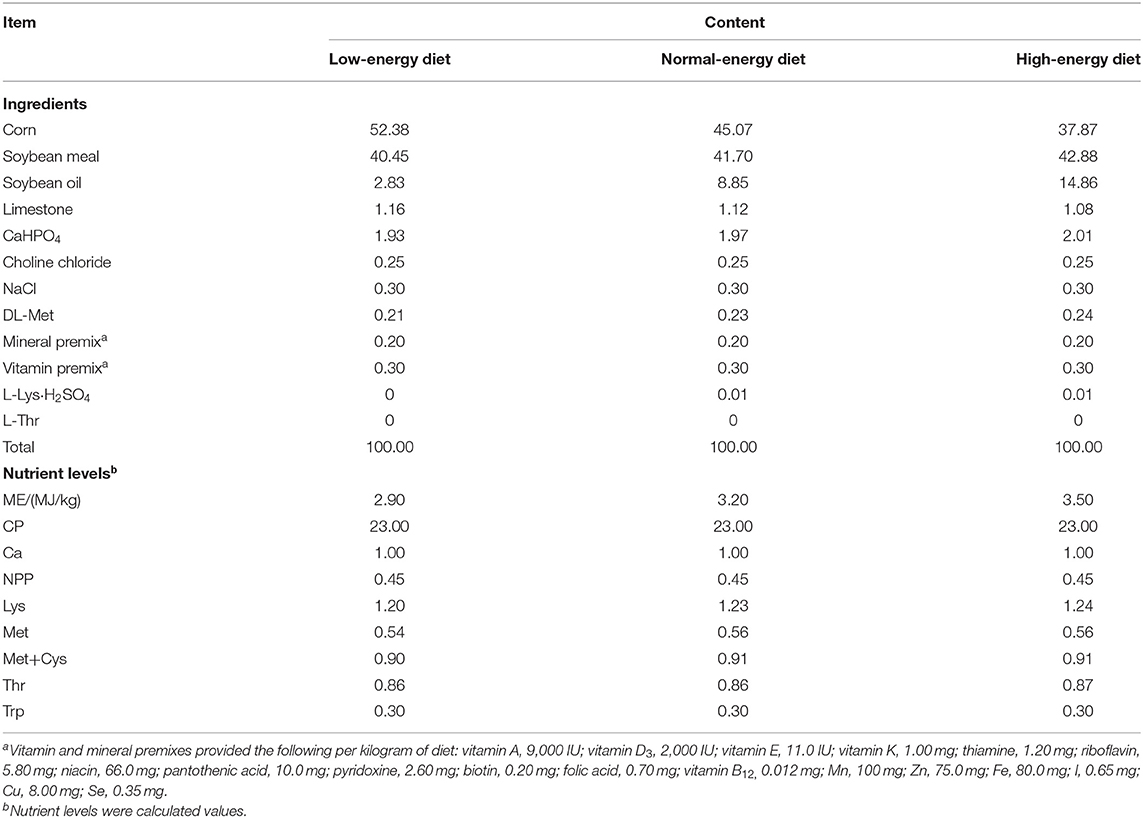
A total of 216 1-day-old Arbor Acres male broiler chicks (Gallus gallus domesticus L.) were obtained from a local hatchery (Da Bao Hatchery, Tai'an, China) and housed in cages in an environmentally controlled room. The brooding temperature was maintained at 35°C for the first 2 days and then gradually decreased by 2–3°C per week, according to the age of broilers (32, 33). The light regime was composed of 23 h of light and 1 h of darkness, and the ambient humidity was 40–50%. The composition and nutrient levels of the chicken diets used in the experiment are listed in Table 1. All birds received feed and water ad libitum during the rearing period. This study was approved by the Shandong Agricultural University and carried out in accordance with the Guidelines for Experimental Animals of the Ministry of Science and Technology (Beijing, China).
Broiler chickens were randomly divided into three groups, with 12 replicates per group and six chickens per replicate. Dietary treatment groups were as follows: (i) low energy diet (LED, ME = 2,900 kcal/kg), (ii) normal energy diet (control, ME = 3,200 kcal/kg), (iii) high energy diet (HED, ME = 3,500 kcal/kg). At 10 days of age, six cages of chickens in each dietary treatment were randomly assigned to receive subcutaneous injections of DEX (2 mg/kg BM/day for 3 days) or sham injection with saline.
Sample Collection and Procedures
At 10 days of age, one chick was selected from each cage for blood sample collection. At the end of the experiment (13 days of age), two chickens from each replicate were selected and sacrificed. Blood samples were obtained from the wing vein of each chicken using a heparinized syringe. Plasma was obtained after centrifugation at 400 g (4°C, 10 min) and stored at −20°C for further analysis (34, 35). After obtaining blood samples, the chickens were slaughtered through the intravenous injection of pentobarbital sodium (30 mg/kg body weight) and jugular exsanguination. The hypothalamus was collected, in accordance with the method described by Liu et al. (36), via a 4–5 mm deep incision, made parallel to the base of the brain (37). Hypothalamus samples were flash-frozen in liquid nitrogen and stored at −80°C.
Growth Performance
The initial body weights of broilers were similar among the three groups (42.63 g in control group, 42.62 g in LED group and 42.52 g in HED group). At 10 and 13 days, broilers in each cage were weighed after 8 h of fasting, feed intake recorded, and replicate recorded values used to calculate the average daily gain (ADG), average daily feed intake (ADFI), and feed conversion rate (FCR) per group.
Plasma Parameters
Plasma concentrations of various factors were measured spectrophotometrically using commercial diagnostic kits (Jiancheng Bioengineering Institute, Nanjing, P. R. China) as follows: total protein (TP; no. A045-2-2), albumin (ALB; no. A028-1-1), glucose (GLU; no. F006), uric acid (UA; no. C012-1-1), total cholesterol (TCHO; no. A111), triglyceride (TG; no. A110), high-density lipoprotein (HDL) cholesterol (no. A112), and low-density lipoprotein (LDL) cholesterol (no. A113). Plasma TP content was determined by Bradford method. Plasma ALB was measured using colorimetric method. Plasma glucose content was measured using the glucose oxidase method. Plasma UA content was determined using the colorimetric method. Plasma TCHO content was measured using the COD-PAP method. Plasma TG content was measured by the GPO-PAP enzymatic method. Plasma HDL-C and LDL were determined according to the kit instructions.
RNA Extraction and Analysis
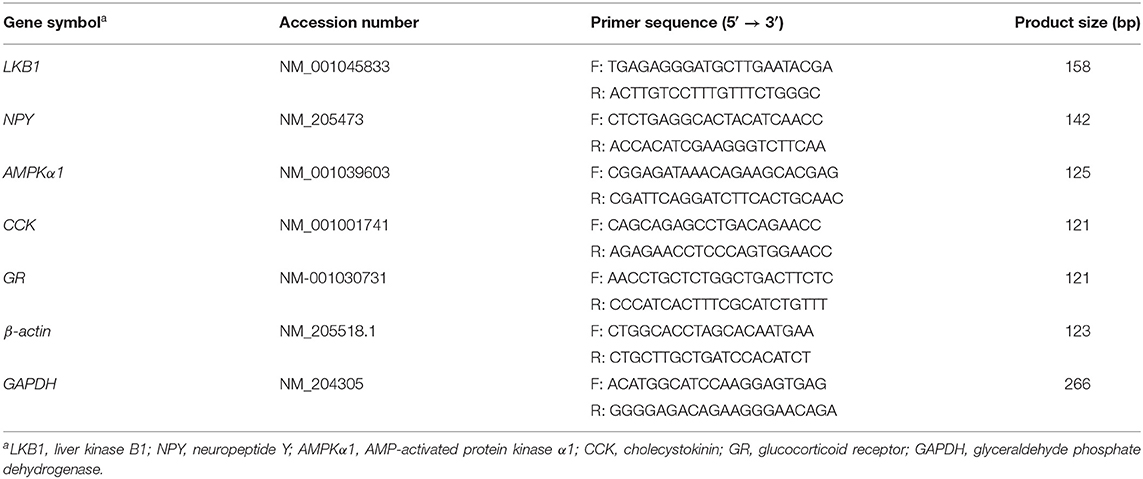
Gene expression in the hypothalamus was quantified by quantitative real-time PCR using total RNA extracted using the Trizol reagent (Invitrogen, San Diego, CA, USA). RNA integrity was assessed through agarose gel electrophoresis. The RNA was quantified by using a DeNovix spectrophotometer (DS-11; DeNovix Inc., Wilmington, DE, USA). RNA purity was verified by determining the absorbance ratio of 260 to 280 nm (OD260/280 = 1.8–2.0). mRNA was reverse transcribed into cDNA using a commercial kit (PrimeScript RT Reagent kit, TaKaRa, Dalian, P.R. China). Real-time PCR was carried out using an Applied Biosystems 7500 Real-time PCR System (Applied Biosystems, Foster, CA, USA). Each RT reaction served as a template in 20 μL PCR reaction mixtures containing 0.2 μmol/L of each primer and SYBR green master mix (Takara, Dalian, Liaoning, and PR China). Primer sequences (Table 2) were designed across exon-intron junctions using the Primer 5.0 software. Reaction conditions were as follows: pre-denaturation at 95°C for 30 s, then 40 cycles of denaturation at 95°C for 5 s, and annealing and extension at 60°C for 34 s. A standard curve was plotted to calculate the efficiency of the real-time PCR primers. Relative mRNA expression levels of genes were calculated using the 2–ΔΔCt method and normalized to the value for β-actin expression. Results were verified using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) levels and levels of the control + saline group were used as a calibrator. All processes were performed in accordance with previously described methods (38–40), and each sample was assayed in triplicate.
Statistical Analysis
Differences between groups receiving different dietary treatments were analyzed using one-way ANOVA and the GLM procedure in Statistical Analysis Systems (SAS) software (version 8.02; SAS Institute Inc., Cary, NC). When differences among individual means were found in ANOVA tests (P < 0.05), means were compared using the Tukey's test. A two-way ANOVA model was used to analyze the main effects of diet energy, DEX treatment, and their interaction using SAS software. Results are presented as mean values and pooled standard error of the mean (SEM). For statistical analysis of ADG and ADFI, one cage was considered as one replicate. For measurement of plasma parameters and gene expression in the hypothalamus, one chick from each cage was sampled, and one replicate comprised one chick. P < 0.05 indicated statistical significance.
Results
Effects of Dietary Energy Levels and DEX on Broiler Chickens Growth Performance (Presented the Baseline Situation, Without DEX, and Then With DEX)
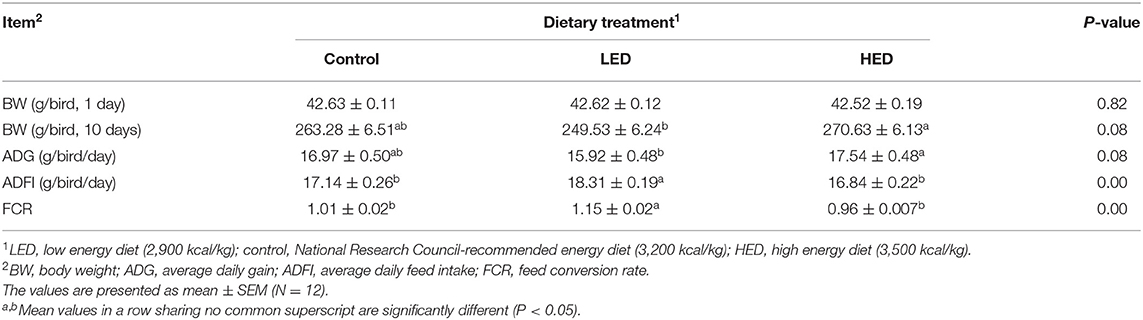
The effects of dietary energy levels on broiler performance are summarized in Table 3. From days 0 to 10, chicks fed with the HED had greater ADG and lower ADFI (P < 0.05) than those fed with the LED, while LED treatment resulted in higher ADFI and FCR (P < 0.05) than the control and HED treatments. The effects of DEX on the performance of broilers fed with different energy diets are presented in Table 4. ADG was decreased in response to DEX injection (P = 0.0002). The control group had a higher ADG and lower FCR than the control-DEX group (P = 0.0003). Further, ADFI in the LED group was lower than that in the control and HED groups (P = 0.0040), irrespective of DEX injection. No significant interaction of diet and DEX treatment on growth performance was detected.

Table 4. Effects of DEX on the performance of 13-day-old broilers fed with different energy level diets1.
Effects of Dietary Energy Level and DEX on Broiler Chickens Plasma Parameters (Presented the Baseline Situation, Without DEX, and Then With DEX)
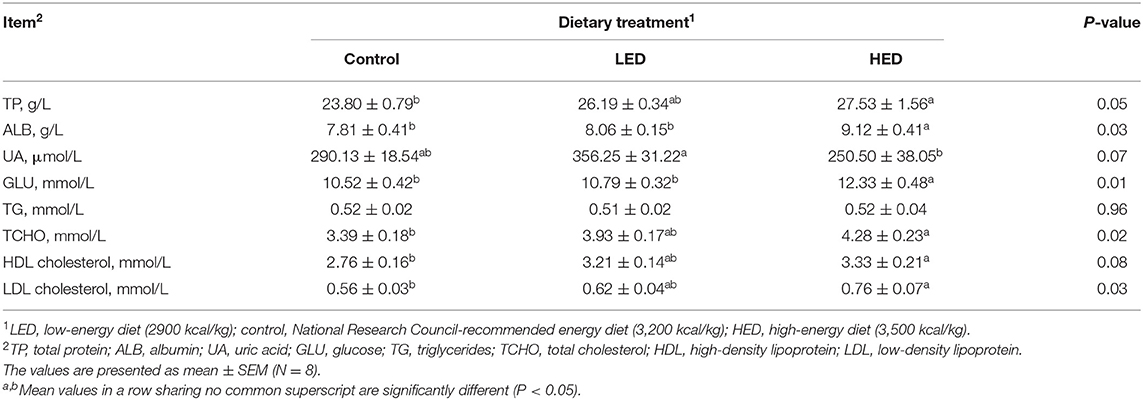
The effects of dietary energy levels on the plasma parameters of broilers are presented in Table 5. HED treatment resulted in higher plasma TP, ALB, GLU, TCHO, HDL, and LDL (P < 0.05) relative to the control treatment group. UA concentration was increased in the LED group compared with that in the HED group (P < 0.05). No significant differences in plasma parameters were observed between the LED and control treatment groups. TG levels were not influenced by dietary treatment. The effects of DEX on the plasma parameters of broilers fed with different energy level diets are shown in Table 6; various plasma parameters were influenced by DEX injection. The contents of TP, ALB, TG, TCHO, HDL, and LDL in the control-DEX group were higher than those in the control group (P < 0.0001, P < 0.0001, P = 0.0740, P < 0.0001, P < 0.0001, and P < 0.0001, respectively). Further, UA content in both the LED and HED groups decreased in response to DEX (P = 0.0101), while the UA content of the LED group was higher than that of the HED group (P = 0.0036). In contrast GLU level was influenced by neither dietary energy level nor DEX injection. An interaction of dietary energy level and DEX injection was found to influence TCHO content (P = 0.0715), while no there was no evidence of an influence of such an interaction on TP, ALB, UA, GLU, TG, HDL, or LDL content.
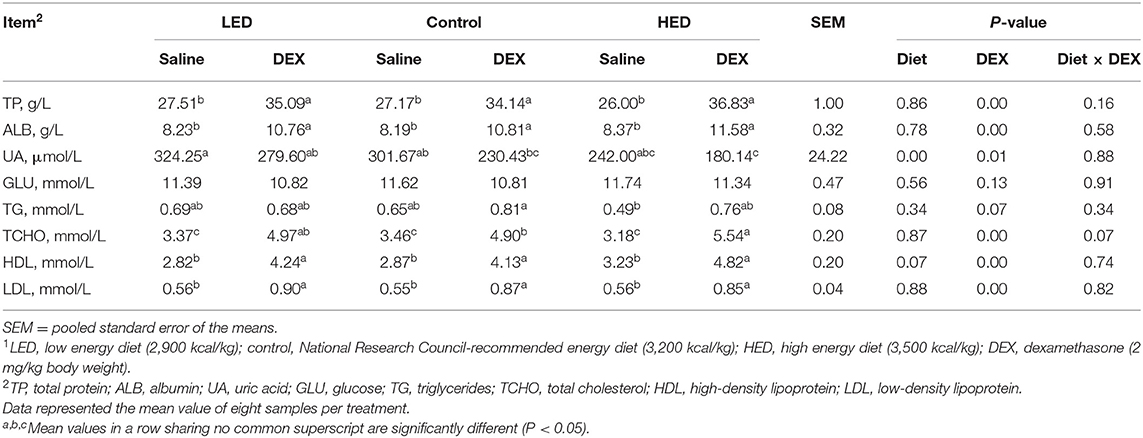
Table 6. Effects of DEX on plasma parameters in 13-day-old broilers fed with different energy level diets1.
Effects of DEX on the Expression Levels of Appetite-Related Genes and AMPK Signaling in Broiler Chickens Fed With Different Energy Level Diet
The relative gene expression levels of AMPK and appetite-related genes are presented in Figures 1, 2, respectively. DEX treatment increased the gene expression levels of LKB1, AMPKα1, NPY, and glucocorticoid receptor (GR) in hypothalamus tissue from 13-day-old broilers (all P < 0.0001). LKB1 mRNA levels in the LED group were higher than those in the HED group (P = 0.0493). In addition, a trend of interaction was observed between DEX and the dietary energy treatments on LKB1 gene expression in the hypothalamus (P = 0.0663). Further, cholecystokinin (CCK) mRNA levels in the hypothalamus of 13-day-old broilers were not affected by either DEX or dietary energy level (P > 0.1).
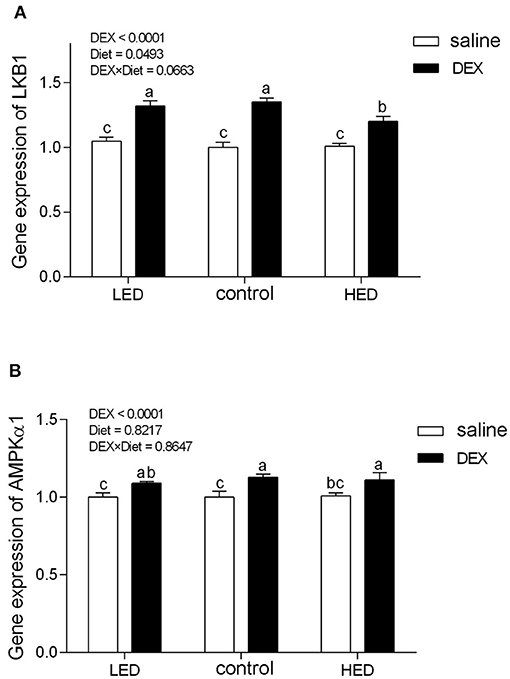
Figure 1. Effects of DEX on the mRNA expression levels of LKB1 (A) and AMPKα1 (B) in the hypothalamus of 13-day-old broilers fed with different energy level diets. Values were obtained from duplicates of each sample and are presented as mean ± SEM (N = 8); a,b,cMeans sharing no common letters differ significantly (P < 0.05; ANOVA). LED, low energy diet; control, National Research Council-recommended diet; HED, high energy diet; DEX, dexamethasone.
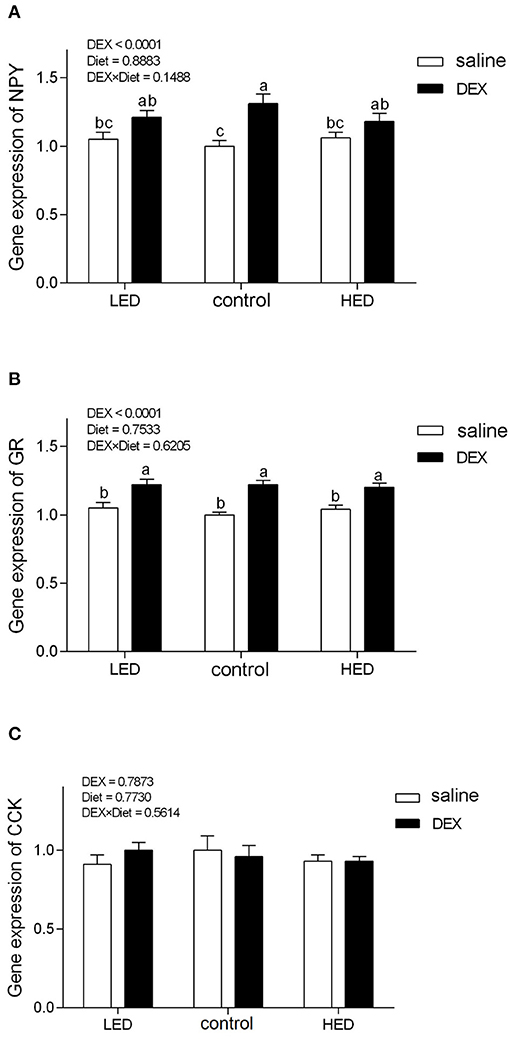
Figure 2. Effects of DEX on the mRNA expression levels of NPY (A), GR (B), and CCK (C) in the hypothalamus of 13-day-old broilers fed with different energy level diets. Values were obtained from duplicates of each sample and are presented as mean ± SEM (N = 8). a,b,cMeans sharing no common letters differ significantly (P < 0.05; ANOVA). LED, low energy diet; control, National Research Council-recommended diet; HED, high energy diet; DEX, dexamethasone.
Discussion
Effects of Dietary Energy Levels and DEX on Broiler Chicken Growth Performance
Dietary energy in broiler nutrition plays a significant and central role in livestock maintenance and production (41). In this study, chicks fed a HED had higher ADG than those fed a LED, consistent with our previous findings (42). The increase in body weight induced by consumption of a HED has been widely verified in human and animal studies (21, 43–45). A LED reduces production performance, due to the low energy intake (46); however, Yuan et al. (1) has found that chicken body weight gain was not altered by dietary energy level. This controversial conclusion may be attributable to the age and the dietary energy level used in the study. Birds consume feed primarily to meet their energy requirements and increase their feed intake in response to dietary energy dilution (41, 47), consistent with our results. Chickens can control their feed intake according to changes in dietary ME concentration (48). The feed intake of broilers fed a low ME diet increased; however, this could not compensate for the poor weight gain and FCR, due to the physical limitations of feed consumption (47). In this study, as dietary energy levels increased, FCR decreased significantly. Several reports have revealed that increased dietary energy can reduce broiler FCR (45, 49, 50). Together, current data suggest that broilers can adjust their feed intake in response to dietary energy levels.
GC can evoke a particular appetite for foods rich in fat or energy (28). DEX had significant effects on ADG and FCR. This observation was consistent with the results of Lv et al. (51), who reported that DEX treatment decreases the body weight gain of broilers as FCR increases. Increased energy expenditure, protein oxidation, and reduced small intestine absorption are responsible for the suppressive effects of GC on growth rate (4, 52, 53). In the present study, the interaction between DEX and dietary energy level had no significant effect on ADG, ADFI, or FCR, similar to the findings of a previous study (4); however, Yuan et al. (1) found that chickens fed a HED had higher BW gain than those receiving a LED on corticosterone treatment, indicating the beneficial effects of a HED. Differences in dietary energy content or fat type may partially account for these discrepancies.
Effects of Dietary Energy Level and DEX on Broiler Chicken Plasma Parameters
Serum GLU is an important energy source conducive to body tissue growth, whereas the serum ALB and TP reflect protein synthesis functions in the broiler liver, which may be associated with physiological status and growth (54). In birds, UA is the main product of nitrogen metabolism, and its content can reflect the direction of protein metabolism (55). In this study, birds fed with HED showed increased levels of plasma GLU, TP, and ALB at 10 days old. GLU in the blood can be oxidized to provide energy and channeled into pathways for fatty acid synthesis (56). The increased GLU concentration detected in the plasma of HED group chickens suggests a high rate of GLU use in fatty acid synthesis. Increased serum ALB and TP can be related to improved protein digestibility and increased availability of amino acid precursors for protein synthesis (57). In this study, at 13 days old, levels of TP and ALB were affected by treatment with DEX, consistent with the result of Lv et al. (51); however, dietary energy levels did not affect these parameters, in accordance with the findings of Yang et al. (4). The level of UA was affected by diet type and DEX treatment; however, no interaction between diet and DEX was evident. These results indicated that dietary energy level cannot alleviate the influence of stress on protein metabolism.
Lipid metabolism was also assessed in our study. Serum TG, TCHO, HDL-cholesterol, and LDL-cholesterol are key indicators of lipid metabolism balance (58–60). Increased levels of serum TCHO and HDL-cholesterol were observed in the HED group, consistent with the results of previous studies (50, 61, 62). HDL-cholesterol is responsible for the uptake of cholesterol from the peripheral tissues and blood and facilitates its transport back to the liver for catabolism (63), whereas LDL-cholesterol has the opposite function (63). Most fatty acids are synthesized in the liver and transported via LDL for storage as TG in adipose tissue (64). Therefore, in the HED group, cholesterol from the peripheral tissues was transported to the liver, as reported by Ge et al. (50), and TG was deposited in the adipose tissue. In the present experiment, DEX treatment significantly increased the levels of TG, TCHO, HDL-C, and LDL-C, regardless of the dietary energy level, as also revealed in previous studies (1, 51). A trend toward an effect of interaction of dietary energy level and DEX treatment on plasma TCHO levels was detected. This finding is consistent with previous research, revealing that the effect of GC on TG is minimal when the diet composition maintains a low lipid flux, but becomes highly significant when the dietary lipid flux increases (65).
Effects of DEX on the Expression Levels of Appetite-Related Genes and AMPK Signaling in Broiler Chickens Fed With Different Energy Level Diets
In the CNS, the hypothalamus is vital in coordinating feed intake and regulating energy homeostasis in mammals and birds (66–68), and various orexigenic and anorexigenic neuropeptides have been identified in the hypothalamus of mammals and poultry (5). Hypothalamic NPY is a potently orexigenic neuropeptide, which can increase appetite in mammals and birds (23, 69, 70). Consistent with previous findings in rats (71) and chicks (28), our data demonstrate that GC treatment increased hypothalamic NPY levels, suggesting that appetite was stimulated. GR, which belongs to the steroid/sterol/thyroid/retinoid/orphan nuclear receptor superfamily, mediates the majority of the known actions of GC (72, 73) and regulates food intake and energy expenditure (22). In this study, GR mRNA levels were increased on treatment with DEX in both dietary energy level groups, indicating that the biological regulation of GC was strengthened. AMPK has emerged as a key molecular regulator with a pivotal role in cellular energy metabolism (74–76). In the CNS, AMPK participates in fasting, inflammation, stress, and other responses (77–80). Once activated, AMPK switches off anabolic pathways (such as fatty acid, TG, and cholesterol syntheses), in favor of catabolic pathways (such as glycolysis and fatty acid oxidation), to sustain energy homeostasis (75, 81). GC activate hypothalamic AMPK activity (82, 83), and this can lead to appetite stimulation (84). In this study, exogenous subcutaneous GC administration significantly increased hypothalamic NPY, AMPKα1, and GR gene expression, while the expression levels of these genes were not altered by dietary energy level; however, level of LKB1 were affected by diet and DEX. LKB1, a kinase upstream of AMPK, has the same function in poultry and mammals (27); LKB1 expression was reduced in DEX-treated chicks fed the HED, indicating that HED can partially reduce activation of AMPK signaling. In other words, high energy diet has the potential to inhibit the activation of central AMPK signaling by stress.
Conclusion
The results of the present study demonstrate that HED improves growth performance, plasma glucose and total cholesterol at 10 days old broilers. Although there is a trend toward an effect of interaction of stress and diet on plasma TCHO content and hypothalamic LKB1 expression, in general, high energy diet has little effect on performance, plasma parameters and central AMPK in 10-day old stressed broiler chickens.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by Guidelines for Experimental Animals of the Ministry of Science and Technology (Beijing, China).
Author Contributions
XH and ZS conceived and designed the experiments and wrote the paper. XH and XL performed the experiments and analyzed the data. LK, CX, and QZ provided essential reagents. All authors read and approved the final manuscript.
Funding
This work was supported by the Natural Science Foundation of Shandong Province (ZR2020MC170), the National Key R&D Program of China (2018YFD0501401-3), and the Shandong Province Agricultural Industry Technology (SDAIT-11-08).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
DEX, dexamethasone; HED, high energy diet; LED, low energy diet; ADG, average daily gain; ADFI, average daily feed intake; FCR, feed conversion rate; TP, total protein; ALB, albumin; GLU, glucose; TCHO, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglycerides; UA, uric acid; HPA, hypothalamic-pituitary-adrenal; GCs, glucocorticoids; CNS, central nervous system; ME, Metabolizable energy; ARC, arcuate nucleus; AgRP, agouti-related peptide; NPY, neuropeptide Y; AMPK, AMP-activated protein kinase; LKB1, liver kinase B1; GR, glucocorticoid receptor; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; CCK, cholecystokinin.
References
1. Yuan L, Lin H, Jiang KJ, Jiao H. C., Song ZG. Corticosterone administration and high-energy feed results in enhanced fat accumulation and insulin resistance in broiler chickens. Br Poult Sci. (2008) 49:487–95. doi: 10.1080/00071660802251731
2. Elenkov IJ, Chrousos GP. Stress Hormones, Th1/Th2 patterns, pro/anti-inflammatory cytokines and susceptibility to disease. Trends Endocrinol Metab. (1999) 10:359–68. doi: 10.1016/S1043-2760(99)00188-5
3. Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. (2003) 3:133–46. doi: 10.1038/nri1001
4. Yang J, Liu L, Sheikhahmadi A. Effects of corticosterone and dietary energy on immune function of broiler chickens. PLoS One. (2015) 10:e0119750. doi: 10.1371/journal.pone.0119750
5. Liu L, Wang X, Jiao H, Zhao J, Lin H. Glucocorticoids inhibited hypothalamic target of rapamycin in high fat diet-fed chicks. Poult Sci. (2015) 94:2221–7. doi: 10.3382/ps/pev168
6. Chrousos GP, Kino T. Glucocorticoid action networks and complex psychiatric and/or somatic disorders. Stress. (2007) 10:213–9. doi: 10.1080/10253890701292119
7. Kino T, Chrousos GP. Glucocorticoid effects on gene expression. Tech Behav Neural Sci. (2005) 15:295–311. doi: 10.1016/S0921-0709(05)80017-3
8. Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. (2007) 91:449–58. doi: 10.1016/j.physbeh.2007.04.011
9. Das TK, Mondal MK, Biswas P, Bairagi B, Samanta CC. Influence of level of dietary inorganic and organic copper and energy level on the performance and nutrient utilization of broiler chickens. Asian-Australas J Anim Sci. (2010) 23:82–9. doi: 10.5713/ajas.2010.60150
10. Alabi OJ, Ng'Ambi JW, Norris D. Dietary energy level for optimum productivity and carcass characteristics of indigenous Venda chickens raised in closed confinement. S Afr J Anim Sci. (2014) 43:75–80. doi: 10.4314/sajas.v43i5.14
11. O'Connor DB, O'Connor RC. Perceived changes in food intake in response to stress: the role of conscientiousness. Stress Health. (2004) 20:279–91. doi: 10.1002/smi.1028
12. Fernandes SS, Koth AP, Parfitt GM. Enhanced cholinergic-tone during the stress induce a depressive-like state in mice. Behav Brain Res. (2018) 347:17–25. doi: 10.1016/j.bbr.2018.02.044
13. Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. (2001) 26:37–49. doi: 10.1016/S0306-4530(00)00035-4
14. Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology. (2004) 145:3754–62. doi: 10.1210/en.2004-0305
15. Coccurello R, Romano A, Giacovazzo G. Increased intake of energy-dense diet and negative energy balance in a mouse model of chronic psychosocial defeat. Eur J Nutr. (2018) 57:1485–98. doi: 10.1007/s00394-017-1434-y
16. Zellner DA, Loaiza S, Gonzalez Z. Food selection changes under stress. Physiol Behav. (2006) 87:789–93. doi: 10.1016/j.physbeh.2006.01.014
17. Auvinen HE, Romijn J.A, Biermasz NR. Effects of high fat diet on the Basal activity of the hypothalamus-pituitary-adrenal axis in mice: a systematic review. Horm Metab Res. (2011) 43:899–906. doi: 10.1055/s-0031-1291305
18. la Fleur SE, Houshyar H, Roy M, Dallman MF. Choice of lard, but not total lard calories, damps adrenocorticotropin responses to restraint. Endocrinology. (2005) 146:2193–9. doi: 10.1210/en.2004-1603
19. Kinzig KP, Hargrave SL, Honors MA. Binge-type eating attenuates corticosterone and hypophagic responses to restraint stress. Physiol Behav. (2008) 95:108–13. doi: 10.1016/j.physbeh.2008.04.026
20. Covasa M, Forbes JM. Selection of foods by broiler chickens following corticosterone administration. Br Poult Sci. (1995) 36:489–501. doi: 10.1080/00071669508417794
21. Wang XJ, Xu SH, Liu L, Song ZG, Jiao HC, Lin H. Dietary fat alters the response of hypothalamic neuropeptide Y to subsequent energy intake in broiler chickens. J Exp Biol. (2016) 220:607–14. doi: 10.1242/jeb.143792
22. Lu Q, Yang Y, Jia S. SRC1 deficiency in hypothalamic arcuate nucleus increases appetite and body weight [published online ahead of print, 2018 Oct 1]. J Mol Endocrinol. (2018) 62:37–46. doi: 10.1530/JME-18-0075
23. Boswell T, Li Q, Takeuchi S. Neurons expressing neuropeptide Y mRNA in the infundibular hypothalamus of Japanese quail are activated by fasting and co-express agouti-related protein mRNA. Brain Res Mol Brain Res. (2002) 100:31–42. doi: 10.1016/S0169-328X(02)00145-6
24. Richards MP. Genetic regulation of feed intake and energy balance in poultry. Poult Sci. (2003) 82:907–16. doi: 10.1093/ps/82.6.907
25. Chen C, Wang H, Jiao H, Wang X, Zhao J, Lin H. Feed habituation alleviates decreased feed intake after feed replacement in broilers. Poult Sci. (2018) 97:733–42. doi: 10.3382/ps/pex358
26. Saha AK, Ruderman NB. Malonyl-CoA and AMP-activated protein kinase: An expanding partnership. Mol Cell Biochem. (2003) 253:65–70. doi: 10.1023/A:1026053302036
27. Proszkowiec-Weglarz M, Richards MP, Ramachandran R, McMurtry JP. Characterization of the AMP-activated protein kinase pathway in chickens. Comp Biochem Physiol B Biochem Mol Biol. (2006) 143:92–106. doi: 10.1016/j.cbpb.2005.10.009
28. Liu L, Wang X, Jiao H. Glucocorticoids induced high fat diet preference via activating hypothalamic AMPK signaling in chicks. Gen Comp Endocrinol. (2017) 249:40–7. doi: 10.1016/j.ygcen.2017.02.018
29. Cai Y, Song Z, Zhang X, Wang X, Jiao H, Lin H. Increased de novo lipogenesis in liver contributes to the augmented fat deposition in dexamethasone exposed broiler chickens (Gallus gallus domesticus). Comp Biochem Physiol C Toxicol Pharmacol. (2009) 150:164–9. doi: 10.1016/j.cbpc.2009.04.005
30. Cai Y, Song Z, Wang X, Jiao H, Lin H. Dexamethasone-induced hepatic lipogenesis is insulin dependent in chickens (Gallus gallus domesticus). Stress. (2011) 14:273–81. doi: 10.3109/10253890.2010.543444
31. Wang X, Lin H, Song Z, Jiao H. Dexamethasone facilitates lipid accumulation and mild feed restriction improves fatty acids oxidation in skeletal muscle of broiler chicks (Gallus gallus domesticus). Comp Biochem Physiol C Toxicol Pharmacol. (2010) 151:447–54. doi: 10.1016/j.cbpc.2010.01.010
32. Song Z, Zhao T, Liu L, Jiao H, Lin H. Effect of copper on antioxidant ability and nutrient metabolism in broiler chickens stimulated by lipopolysaccharides. Arch Anim Nutr. (2011) 65:366–75. doi: 10.1080/1745039X.2011.609753
33. Liu SQ, Zhao JP, Fan XX. Rapamycin, a specific inhibitor of the target of rapamycin complex 1, disrupts intestinal barrier integrity in broiler chicks. J Anim Physiol Anim Nutr (Berl). (2016) 100:323–30. doi: 10.1111/jpn.12375
34. Huang C, Jiao H, Song Z, Zhao J, Wang X, Lin H. Heat stress impairs mitochondria functions and induces oxidative injury in broiler chickens. J Anim Sci. (2015) 93:2144–53. doi: 10.2527/jas.2014-8739
35. Zhao JP, Cui DP, Zhang ZY, Jiao HC, Song ZG, Lin H. Live performance, carcass characteristic and blood metabolite responses of broilers to two distinct corn types with different extent of grinding. J Anim Physiol Anim Nutr (Berl). (2017) 101:378–88. doi: 10.1111/jpn.12451
36. Liu L, Xu S, Wang X, Jiao H, Lin H. Peripheral insulin doesn't alter appetite of broiler chicks. Asian-Australas J Anim Sci. (2016) 29:1294–9. doi: 10.5713/ajas.15.0674
37. Higgins SE, Ellestad LE, Trakooljul N. Transcriptional and pathway analysis in the hypothalamus of newly hatched chicks during fasting and delayed feeding. BMC Genomics. (2010) 11:162. doi: 10.1186/1471-2164-11-162
38. Tang D, Wu J, Jiao H, Wang X, Zhao J, Lin H. The development of antioxidant system in the intestinal tract of broiler chickens. Poult Sci. (2019) 98:664–678. doi: 10.3382/ps/pey415
39. Uerlings J, Song ZG, Hu XY. Heat exposure affects jejunal tight junction remodeling independently of adenosine monophosphate-activated protein kinase in 9-day-old broiler chicks. Poult Sci. (2018) 97:3681–90. doi: 10.3382/ps/pey229
40. Zhao JP, Bao J, Wang XJ, Jiao HC, Song ZG, Lin H. Altered gene and protein expression of glucose transporter1 underlies dexamethasone inhibition of insulin-stimulated glucose uptake in chicken muscles. J Anim Sci. (2012) 90:4337–45. doi: 10.2527/jas.2012-5100
41. Zhao PY, Kim IH. Effect of diets with different energy and lysophospholipids levels on performance, nutrient metabolism, and body composition in broilers. Poult Sci. (2016) 96:1341–7. doi: 10.3382/ps/pew469
42. Hu X, Wang Y, Sheikhahmadi A. Effects of dietary energy level on appetite and central adenosine monophosphate-activated protein kinase (AMPK) in broilers. J Anim Sci. (2019) 97:4488–95. doi: 10.1093/jas/skz312
43. West DB, York B. Dietary fat, genetic predisposition, and obesity: lessons from animal models. Am J Clin Nutr. (1998) 67:505S−12S. doi: 10.1093/ajcn/67.3.505S
44. Murtaugh MA, Herrick JS, Sweeney C. Diet composition and risk of overweight and obesity in women living in the southwestern United States. J Am Diet Assoc. (2007) 107:1311–21. doi: 10.1016/j.jada.2007.05.008
45. Niu ZY, Shi JS, Liu FZ, Wang XH, Gao CQ, Yao LK. Effects of dietary energy and protein on growth performance and carcass quality of broilers during starter phase. Int J Poult Sci. (2009) 8:508–11. doi: 10.3923/ijps.2009.508.511
46. Wang JP, Zhang ZF, Yan L, Kim IH. Effects of dietary supplementation of emulsifier and carbohydrase on the growth performance, serum cholesterol and breast meat fatty acids profile of broiler chickens. Anim Sci J. (2015) 87:250–6. doi: 10.1111/asj.12412
47. Saxena R, Saxena VK, Tripathi V. Dynamics of gene expression of hormones involved in the growth of broiler chickens in response to the dietary protein and energy changes. Gen Comp Endocrinol. (2020) 288:113377. doi: 10.1016/j.ygcen.2019.113377
48. Leeson S, Caston L, Summers JD. Broiler response to diet energy. Poult Sci. (1996) 75:529–35. doi: 10.3382/ps.0750529
49. Maiorka A, Dahlke F, Santin E, Kessler A, Penz AM Jr. Effect of energy levels of diets formulated on total or digestible amino acid basis on broiler performance. Rev Bras Cienc Avic. (2004) 6:87–91. doi: 10.1590/S1516-635X2004000200003
50. Ge XK, Wang AA, Ying ZX. Effects of diets with different energy and bile acids levels on growth performance and lipid metabolism in broilers. Poult Sci. (2019) 98:887–95. doi: 10.3382/ps/pey434
51. Lv ZP, Peng YZ, Zhang BB, Fan H, Liu D, Guo YM. Glucose and lipid metabolism disorders in the chickens with dexamethasone-induced oxidative stress. J Anim Physiol Anim Nutr (Berl). (2018) 102:e706–e17. doi: 10.1111/jpn.12823
52. Dong H, Lin H, Jiao HC, Song ZG, Zhao JP, Jiang KJ. Altered development and protein metabolism in skeletal muscles of broiler chickens (Gallus gallus domesticus) by corticosterone. Comp Biochem Physiol A Mol Integr Physiol. (2007) 147:189–95. doi: 10.1016/j.cbpa.2006.12.034
53. Hu XF, Guo YM, Huang BY, Bun S, Zhang LB, Li JH. The effect of glucagon-like peptide 2 injection on performance, small intestinal morphology, and nutrienttransporter expression of stressed broiler chickens. Poult Sci. (2010) 89:1967–74. doi: 10.3382/ps.2009-00547
54. Limdi JK, Hyde GM. Evaluation of abnormal liver function tests. Postgrad Med J. (2003) 79:307–12. doi: 10.1136/pmj.79.932.307
55. Okumura JI, Tasaki I. Effect of fasting, refeeding and dietary protein level on uric acid and ammonia content of blood, liver and kidney in chickens. J Nutr. (1969) 97:316–20. doi: 10.1093/jn/97.3.316
56. Uyeda K, Repa JJ. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metab. (2006) 4:107–10. doi: 10.1016/j.cmet.2006.06.008
57. Prola L, Nery J, Lauwaerts A. Effects of N,N-dimethylglycine sodium salt on apparent digestibility, vitamin E absorption, and serum proteins in broiler chickens fed a high- or low-fat diet. Poult Sci. (2013) 92:1221–6. doi: 10.3382/ps.2012-02465
58. Helkin A, Stein JJ, Lin S, Siddiqui S, Maier KG, Gahtan V. Dyslipidemia part 1–review of lipid metabolism and vascular cell physiology. Vasc Endovascular Surg. (2016) 50:107–18. doi: 10.1177/1538574416628654
59. Song D, Wang YW, Hou YJ. The effects of dietary supplementation of microencapsulated Enterococcus faecalis and the extract of Camellia oleifera seed on growth performance, immune functions, and serum biochemical parameters in broiler chickens1. J Anim Sci. (2016) 94:3271–7. doi: 10.2527/jas.2016-0286
60. Lai W, Huang W, Dong B. Effects of dietary supplemental bile acids on performance, carcass characteristics, serum lipid metabolites and intestinal enzyme activities of broiler chickens. Poult Sci. (2018) 97:196–202. doi: 10.3382/ps/pex288
61. Siritarino PW. Effects of diet on high-density lipoprotein cholesterol. Curr Atheroscler Rep. (2011) 13:453–60. doi: 10.1007/s11883-011-0207-y
62. Khan TJ, Kuerban A, Razvi SS. In vivo evaluation of hypolipidemic and antioxidative effect of ‘Ajwa’ (Phoenix dactylifera L.) date seed-extract in high-fat diet-induced hyperlipidemic rat model. Biomed Pharmacother. (2018) 107:675–80. doi: 10.1016/j.biopha.2018.07.134
63. Miller GJ, Miller NE. Plasma-high-density-lipoprotein concentration and development of ischaemic heart-disease. Lancet. (1975) 1:16–9. doi: 10.1016/S0140-6736(75)92376-4
64. Hermier D. Lipoprotein metabolism and fattening in poultry. J Nutr. (1997) 127:S805–S8. doi: 10.1093/jn/127.5.805S
65. Mantha L, Palacios E, Deshaies Y. Modulation of triglyceride metabolism by glucocorticoids in diet-induced obesity. Am J Physiol. (1999) 277:R455–R64. doi: 10.1152/ajpregu.1999.277.2.R455
66. Denis RG, Joly-Amado A, Cansell C. Central orchestration of peripheral nutrient partitioning and substrate utilization: implications for the metabolic syndrome. Diabetes Metab. (2014) 40:191–7. doi: 10.1016/j.diabet.2013.11.002
67. Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. (2006) 443:289–95. doi: 10.1038/nature05026
68. Kuenzel WJ, Beck MM, Teruyama R. Neural sites and pathways regulating food intake in birds: a comparative analysis to mammalian systems. J Exp Zool. (1999) 283:348–64. doi: 10.1002/(SICI)1097-010X(19990301/01)283:4/5<348::AID-JEZ5>3.0.CO
69. Kalra SP, Dube MG, Sahu A, Phelps CP, Kalra PS. Neuropeptide Y secretion increases in the paraventricular nucleus in association with increased appetite for food. Proc Natl Acad Sci U S A. (1991) 88:10931–5. doi: 10.1073/pnas.88.23.10931
70. Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci. (1998) 1:271–2. doi: 10.1038/1082
71. Larsen PJ, Jessop DS, Chowdrey HS, Lightman SL, Mikkelsen JD. Chronic administration of glucocorticoids directly upregulates prepro-neuropeptide Y and Y1-receptor mRNA levels in the arcuate nucleus of the rat. J Neuroendocrinol. (1994) 6:153–9. doi: 10.1111/j.1365-2826.1994.tb00566.x
72. Kino T, De Martino MU, Charmandari E, Mirani M, Chrousos GP. Tissue glucocorticoid resistance/hypersensitivity syndromes. J Steroid Biochem Mol Biol. (2003) 85:457–67. doi: 10.1016/S0960-0760(03)00218-8
73. Chrousos GP, Kino T. Intracellular glucocorticoid signaling: a formerly simple system turns stochastic. Sci STKE. (2005) 304:pe48. doi: 10.1126/stke.3042005pe48
74. Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. (2005) 1:15–25. doi: 10.1016/j.cmet.2004.12.003
75. Scerif M, Füzesi T, Thomas JD. CB1 receptor mediates the effects of glucocorticoids on AMPK activity in the hypothalamus. J Endocrinol. (2013) 219:79–88. doi: 10.1530/JOE-13-0192
76. Spasic MR, Callaerts P, Norga KK. AMP-activated protein kinase (AMPK) molecular crossroad for metabolic control and survival of neurons. Neuroscientist. (2009) 15:309–16. doi: 10.1177/1073858408327805
77. Lim CT, Kola B, Korbonits M. AMPK as a mediator of hormonal signalling. J Mol Endocrinol. (2010) 44:87–97. doi: 10.1677/JME-09-0063
78. Liu F, Benashski SE, Persky R, Xu Y, Li J, McCullough LD. Age-related changes in AMP-activated protein kinase after stroke. Age (Dordr). (2012) 34:157–68. doi: 10.1007/s11357-011-9214-8
79. Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. (2004) 428:569–74. doi: 10.1038/nature02440
80. Yi CO, Jeon BT, Shin HJ, Jeong EA, Chang KC, Lee JE. Resveratrol activates AMPK and suppresses LPS-induced NF-κB-dependent COX-2 activation in RAW 264.7 macrophage cells. Anat Cell Biol. (2011) 44:194–203. doi: 10.5115/acb.2011.44.3.194
81. Viollet B, Andreelli F, Jorgensen SB, Perrin C, Flamez D, Mu J. Physiological role of AMP-activated protein kinase (AMPK): insights from knockout mouse models. Biochem Soc Trans. (2003) 31:216–9. doi: 10.1042/bst0310216
82. Christ-Crain M, Kola B, Lolli F. AMP-activated protein kinase mediates glucocorticoid-induced metabolic changes: a novel mechanism in Cushing's syndrome. FASEB J. (2008) 22:1672–83. doi: 10.1096/fj.07-094144
83. Shimizu H, Arima H, Watanabe M, Goto M, Banno R, Sato I, et al. Glucocorticoids increase neuropeptide Y and agouti-related peptide gene expression via adenosine monophosphateactivated protein kinase signaling in the arcuate nucleus of rats. Endocrinology. (2008) 149:4544–53. doi: 10.1210/en.2008-0229
Keywords: broiler, diet energy level, stress, appetite, AMPK
Citation: Hu X, Li X, Xiao C, Kong L, Zhu Q and Song Z (2021) Effects of Dietary Energy Level on Performance, Plasma Parameters, and Central AMPK Levels in Stressed Broilers. Front. Vet. Sci. 8:681858. doi: 10.3389/fvets.2021.681858
Received: 17 March 2021; Accepted: 05 May 2021;
Published: 28 May 2021.
Edited by:
Xi He, Hunan Agricultural University, ChinaReviewed by:
F. Capela e Silva, University of Evora, PortugalHaijun Zhang, Feed Research Institute (CAAS), China
Copyright © 2021 Hu, Li, Xiao, Kong, Zhu and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhigang Song, naposong@qq.com
†These authors have contributed equally to this work and share first authorship
 Xiyi Hu
Xiyi Hu Xianlei Li
Xianlei Li Chuanpi Xiao
Chuanpi Xiao Linglian Kong
Linglian Kong Qidong Zhu
Qidong Zhu Zhigang Song
Zhigang Song