Long-Term Variability of Piscivorous Fish in China Seas Under Climate Change With Implication for Fisheries Management
- 1Key Laboratory of Mariculture, Ministry of Education, Ocean University of China, Qingdao, China
- 2Frontiers Science Center for Deep Ocean Multispheres and Earth System (FDOMES), Ocean University of China, Qingdao, China
- 3Laboratory for Marine Fisheries Science and Food Production Processes, Pilot National Laboratory for Marine Science and Technology, Qingdao, China
- 4Fisheries and Oceans Canada, Pacific Biological Station, Nanaimo, BC, Canada
- 5Department of Environmental Biology and Fisheries Science, National Taiwan Ocean University, Keelung, Taiwan
- 6CSIRO Oceans and Atmosphere, Brisbane, QLD, Australia
Due to persistent fishing expansion in the China Seas over the past six decades, fisheries resources have been over-exploited; as a result, exploited fish have become smaller in size and younger in age. Marine piscivorous fish constituted a large portion of Chinese fisheries catch, long-term variability of which has rarely been investigated despite intense fishing pressure and climate change. In this study, we attempt to identify their responses to climate change and fishing activities and to provide scientific basis for sustainable exploitation of these resources. Seven taxa from pelagic to demersal species inhabiting either cold-water or warm-water were selected to represent the piscivorous fish assemblage in the China Seas. Total catch of these piscivorous fish in the China Seas increased during the early 1990s, stabilizing around 1.2 million tons after 1997. Principal component analysis (PCA) showed evident interannual-decadal variabilities in the catch of these fish with step changes around 1985/86 and 1997/98. Individual taxa, however, showed different trends in catches with sharks, rays, and lizardfishes manifesting downward trends while Pacific cod, eels, and hairtail increasing. Common dolphinfish and Japanese-Spanish mackerel increased largely in the 1990s but declined slightly during the 2000s. Although there were temporal overlaps between climate change and fishing variabilities, results of gradient forest analyses indicated that fishing effort imposed the most important influence on piscivorous fish. And among all climate variables explored in this study, sea surface temperature (SST) especially that of the East China Sea, had greatest impacts on variations in piscivorous fish catch, which may have been gradually exacerbated by the continued high fishing intensity. In addition, significant changes were identified in the life history traits in the species we evaluated, such as reduced average body sizes and truncated age compositions, strongly indicating the effect of fishing. We therefore advocate precautionary fishery practices under climate change.
Introduction
Monitoring and understanding long-term variabilities in marine ecosystems have become global concern (Ding et al., 2016, 2017). Changes in marine ecosystems and fisheries resources have been documented widely (Butchart et al., 2010), and multiple climate-related and anthropogenic drivers of such changes have been identified (Christensen et al., 2003; Halpern et al., 2008).
Marine ecosystems respond to climate changes over a wide range of spatial and temporal scales. Climate variability affects primary up to tertiary productivity by controlling nutrient supply (Frederiksen et al., 2006). Climate changes directly influence larval survival and recruitment of fishes (Ottersen et al., 2006) and can impact population dynamics of marine organisms indirectly through food web structure under “top-down” controls. Among multiple climate variables, sea temperature has received the most attention. Rapid warming has been observed for large marine ecosystems in the world oceans, particularly for the East Asian Seas including the East China Sea and Sea of Japan (Belkin, 2009). The warming trend is strongly modifying the key marine ecological processes (Bennett et al., 2015), as well as rapidly breaking down long-standing biogeographic boundaries and causing species redistribution at a global scale (Hobday et al., 2015), which consequently contributes to the erosion of ecosystem resilience (Graham et al., 2015). And a recent study indicates that historical warming has large impacts on marine fisheries production (Free et al., 2019).
Marine piscivorous fish are top predators that have long played important roles both in marine ecosystems, influencing ecosystem structure through their top-down process (Hobday et al., 2015), and in fisheries (Myers and Worm, 2005). Since piscivorous fish at higher trophic level have relatively longer life spans reflecting longer term trends (Thompson et al., 2012), altered dynamics of these species may signal lasting change in bottom-up forcing of marine ecosystems (Goedegebuure et al., 2017). Marine piscivorous fish are of considerable economic importance, providing valuable revenue for many countries. However, some estimates indicated that piscivorous fish communities worldwide have been depleted by 90% during the last 50 years (Myers and Worm, 2003), which may have large impacts on their role in marine ecosystems as indicated by “the effect of fishing down marine food-webs” (Pauly et al., 1998). Therefore, research on marine piscivorous fish is important for sustainable management of marine fisheries and ecosystems.
Marine fisheries have undergone significant variations over the past six decades. At present, about one-third of global fishery stocks is overexploited or collapsed and fishing has been regarded as one of the greatest pressures on marine ecosystems (Costello et al., 2010) since it not only reduces populations of target species but also alters ecosystem structures. Recent researches suggest that amplification of fish populations’ response to climate, possibly as a result of exploitation, is already at play in the ocean (Ottersen et al., 2006). Indeed, fishing can magnify the fluctuations in fish abundance, impact substantially population dynamics and cause change in size structure of fish stocks in tandem with climate variations (Jackson et al., 2001; Hsieh et al., 2006; Tu et al., 2018). Since marine fisheries are concurrently impacted by fishing and climate variability, assessments of the single and combined effects are required to achieve better understanding of climate and fishing influence.
The China Seas are located at the western margin of the North Pacific, consisting of the Bohai Sea (BS), the Yellow Sea (YS), the East China Sea (ECS) and the South China Sea (in the following China Seas refer to the seas including BS, YS, and ECS) (Figure 1). China Seas belong to one of the most productive fishing areas in the world oceans. The relatively abundant resources in the area and the large fishing efforts have turned China into the country with the largest fishery production in the world since 1989, and Chinese marine catch comprises about 20% of the total world catch (FAO, 2014). However, although ranking among the top in the world, Chinese marine fisheries resources are severely overexploited and already show signs of recession with great declines of CPUE (catch per unit effort, a relative abundance index of fish stocks) since the 1960s under decades of high fishing pressure (Yan et al., 2005; Ma et al., 2019).
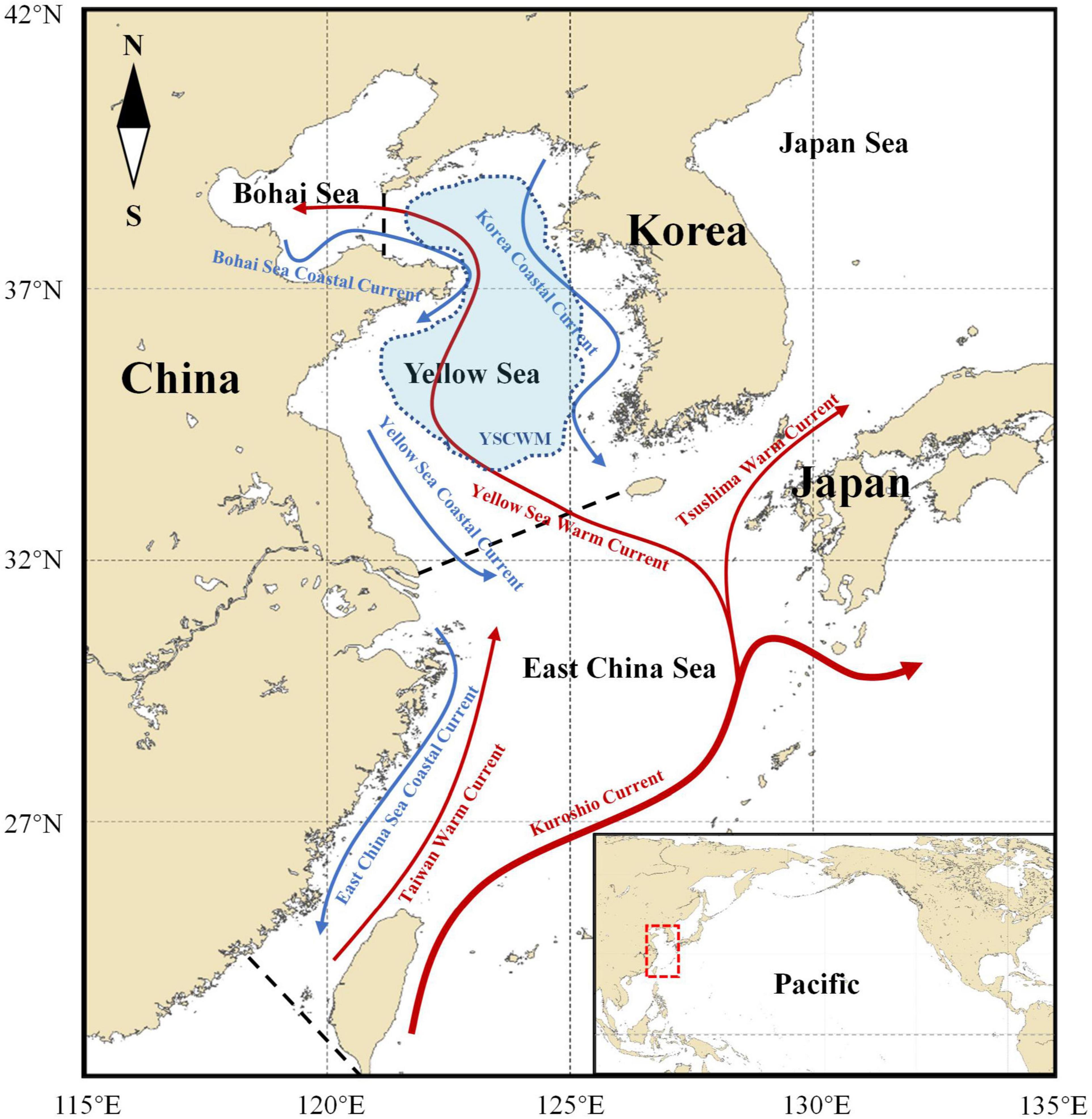
Figure 1. Location of China Seas (Bohai Sea, Yellow Sea, and East China Sea) and the schematic illustration of their currents. Dashed red box in the bottom right corner map shows the China Seas which is in the western margin of the North Pacific. Arrows with solid lines represent the currents. Warm currents: red. Cold currents: blue. Blue shading represents the yellow sea cold water mass (YSCWM). Dashed black lines are boundaries between seas.
Piscivorous fish such as Japanese-Spanish mackerel (Scomberomorus niphonius) constitute an important part of the China Seas marine ecosystem, and contribute to a large part of total catch with high economic values. However, holistic information including abundance trend, changes in life history traits, and responses to climate variability remains limited. In the over-exploited situation, longer-lived demersal and predatory pelagic species may be replaced by lower-trophic-level species due to the “fishing down marine food webs” effect (Pauly et al., 1998). In addition, previous studies have demonstrated that the warming sea surface temperature (SST) in the China Seas with interdecadal fluctuation corresponded well to regime shifts in the North Pacific, and can be divided into three stages, including cold period (1960–1982), warming period (1983–1998) and warm period (1999–2011) (Ma et al., 2019). Aside from fishing impacts, changing patterns of small pelagic fish and cephalopods in the overexploited China Seas are strongly influenced by these climate regime shifts (Pang et al., 2018; Ma et al., 2019). And it is also found that during the warm period, the resource index of demersal fish and large predators decreased, while that of mesopelagic fish and invertebrates increased (Yuan et al., 2017). Therefore, to facilitate relevant research on Chinese fishery resources, understanding the variations and change mechanisms of piscivorous fish is essential and urgent.
We hypothesize that because of their different thermal/habitat preferences and life history traits, piscivorous fish in the China Seas may have been impacted by high fishing pressure and climate change differently. How fishing and oceanographic/climatic changes have impacted piscivorous fish at community level and how piscivorous fish have responded to the dual pressures has hitherto not been investigated in the China Seas. In this study, we for the first time fully utilized all information and data available to us, including long-term fisheries catch data from Chinese piscivore fisheries as well as biological data such as body-length, weight and age structure. We aim to (1) demonstrate long-term trends and changes in fisheries catch and life history traits of piscivorous fish, (2) identify responses of fisheries catch to joint effects of fishing and climate variability, and (3) provide scientific basis for fisheries management and sustainable exploitation of these resources.
Materials and Methods
Selection of Piscivorous Fish
In the China Seas, there are over 20 species of piscivorous fish, most of which are commercially important and play key roles in the China Seas marine ecosystems (Zhang et al., 2007a, b). In particular, Japanese-Spanish mackerel and hairtail (maily Trichiurus japonicus) are two species that contribute substantially to the overall Chinese fisheries catches. These piscivorous fish mainly feed on small pelagic fishes (Liu et al., 2009; Jin et al., 2010; Wan et al., 2010; Yan et al., 2010; An et al., 2012; Li et al., 2012), such as anchovies (e.g., Engraulis japonicus, Setipinna taty), sardines (e.g., Sardinops melanostictus, Sardinella zunasi), scads (e.g., Decapterus maruadsi, Trachurus japonicus) and mackerels (e.g., Scomber japonicus, S. australasicus). Different piscivorous fish in the China Seas occupy different habitats, including demersal, benthopelagic, pelagic, pelagic-oceanic, and reef-associated habitats. Thus we selected a number of piscivorous fish encompassing all different habitat types and merged them into seven taxa, including Pacific cod (Gadus macrocephalus), hairtail, Japanese-Spanish mackerel, marine eel (e.g., Muraenesox cinereus), sharks and rays (e.g., Sphyrna lewini, Isurus oxyrinchus, Rhinobatos schlegeli), lizardfishes (Saurida), and common dolphinfish (Coryphaena hippurus). Pacific cod is uniquely a cold-water species dwelling in Yellow Sea Cold Water Mass with relatively low water temperature below 10°C. All others are warm-water taxa that spread in the China Seas with an average water temperature over 15°C.
Fishery and Biological Data
Fisheries annual catch data of the piscivorous fish were mainly from Chinese Fishery Statistics for the period of 1955–2019 (Zhao et al., 2020). Missing values were supplemented by the fishery data in the Exclusive Economic Zone (EEZ) of China from the Sea Around Us project1 (Pauly and Zeller, 2016), which were comparable with those from Japan, Taiwan and the FAO Global Capture Production (1955–2019) dataset (FAO-FIGIS, 2019; Table 1).
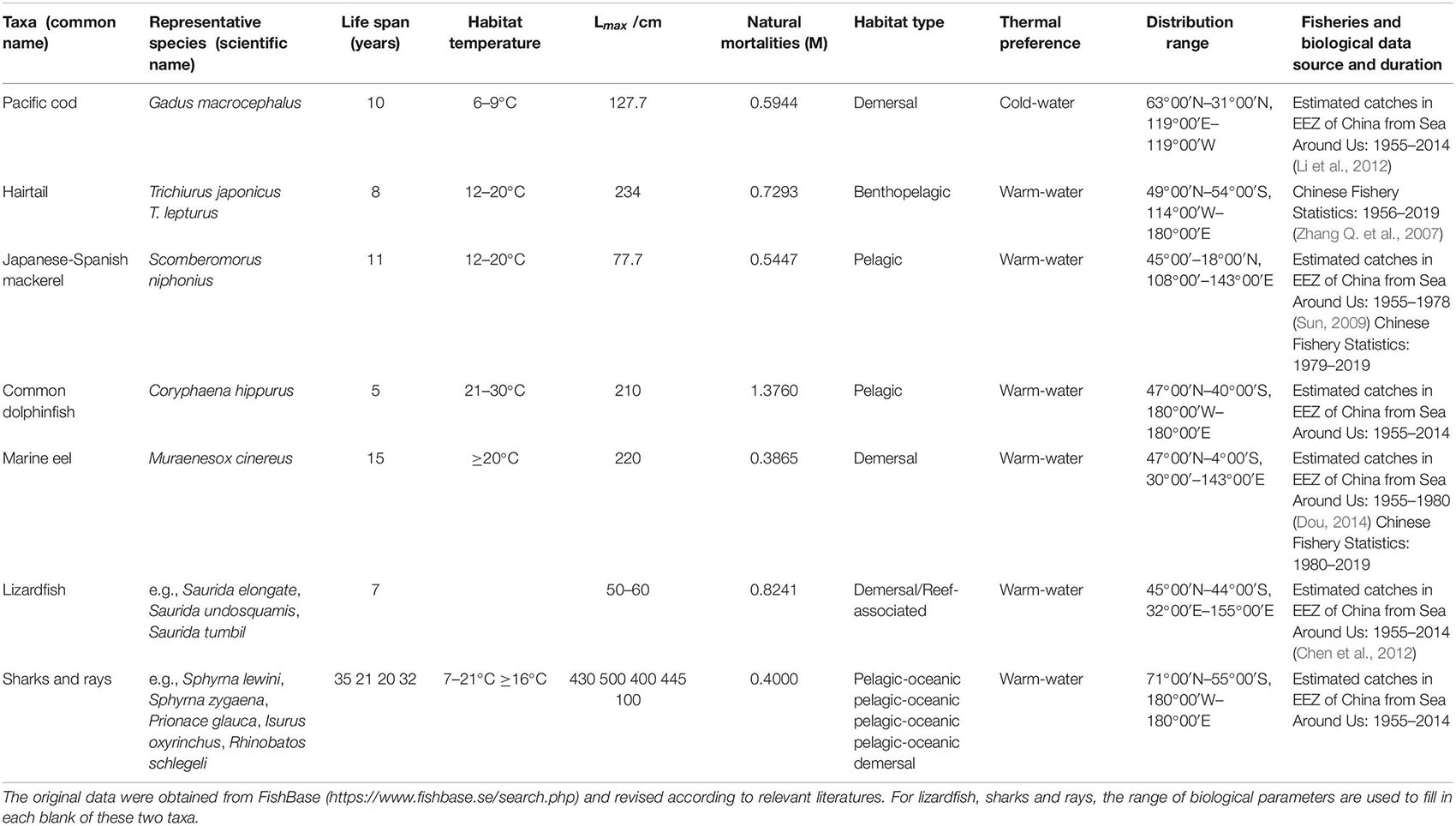
Table 1. Ecological and fisheries characteristics for the seven piscivorous fishes in the China Seas.
Fishing effort data for the period of 1955–2019 including the number of marine fishing boats and engine power in the China Seas were also derived from the Chinese Fishery Statistics. The CPUE time series of piscivorous fish were estimated as metric tons of annual catch per boat or per 100 kW; however, they were regarded only as references for the abundance of fishery resources and were not used for quantitative analysis for lack of accurate fishing effort data.
Biological data of piscivorous fish from the China Seas, including body-length, weight, and age structure, were compiled from related literatures published in China for detecting variations in their life history traits (Table 1).
Estimation of Biomass Index
The optimized catch-only method (OCOM; Zhou et al., 2018), as a supplementary method to improve fisheries data quality in this study, was applied to the catch data to estimate the biomass of these piscivorous fish in the China Seas. OCOM is mainly based on a Graham-Schaefer surplus production model to estimate stock depletion (d) or stock saturation S = 1 − d, being enhanced by incorporating priors from the relationship between natural mortality (M) and fishing mortality at maximum sustainable yield (i.e., FMSY), a boosted regression trees (BRT) model and an algorithm to search for feasible parameter values (Zhou et al., 2018). The main equation is written as:
where Bt and Ct are biomass and fisheries catch at the beginning of time step t (year), r is the intrinsic growth rate, K is the carrying capacity (equal to the unfished or initial biomass B0 for a surplus production model). The parameter K can be solved by available prior information on r and S (S = Bt/K) (Zhou et al., 2017).
Basically, the implementation of OCOM involves: (i) drawing a large number (e.g., n = 10,000) of values for r and S from their priors; (ii) deriving K by solving Be/K = S (Be is the biomass at the end of the time series) using the optimization algorithm (function “optimize” in R; R Core Team, 2018); and (iii) computing any output quantities of interest. Especially note that OCOM assumes that the biomass is less than K for all years, and as with other catch-only methods, it is very sensitive to the priors, particularly that for depletion (d) (Wetzel and Punt, 2015; Zhou et al., 2018).
For each taxon, the population dynamics equation is also written as:
where Rt + Gt (the sum of recruitment and biomass growth) is regarded as one parameter representing the available population growth (At). Biomass loss due to natural mortality M (i.e., Dt) is calculated as:
As true M may not be available for many data-poor species, we used an empirical life-history invariant equation of Then et al. (2015) to approximate M for each of the seven piscivorous taxa:
where tmax is the maximum age.
Thus, the OCOM provides biomass estimates over time, which are then used, alongside with fisheries catch, to examine linkages with fishing intensity and environmental variables. In addition, in order to differentiate the effect of climate change from fishing, we used the ratio of available population growth to biomass (i.e., At/Bt, denoted as RAPGt), a relative index of population variability for the piscivorous fish, to represent environmental effect on variability in the piscivorous fish.
Climate Indices and Oceanographic Data
To associate the dynamics of the piscivorous fish with climatic and oceanographic conditions, we selected PDO (Pacific Decadal Oscillation), NPI (North Pacific Index), AOI (Arctic Oscillation Index), SOI (Southern Oscillation Index), and MOI (Asian Monsoon Index) as basin-scale climatic indices for the North Pacific. These climatic indices are well-documented and largely associated with variations in marine ecosystems and fish communities in the North Pacific (e.g., Tian et al., 2008, 2014). In addition to the basin climatic indices, we also used region-scale SST and sea surface salinity (SSS) time series in the China Seas to explore the regional marine thermohaline variations and their relationships with piscivorous fish catch. SSTs are obtained from the Met Office Hadley Centre observations datasets2, which consist of monthly grid data with a resolution of one degree (latitude × longitude) for the period of 1955–2016. And SSSs are based on the Simple Ocean Data Assimilation (SODA) Reanalysis3 consisting of monthly grid data with a resolution of 0.5 degree (latitude × longitude) for the period of 1958–2008. Both seasonal average SSTs and SSSs for summer (July to September) and winter (January to March) were calculated for different regions of the China Seas (Ma et al., 2019).
Statistical Analysis
Sequential t-test analyses of regime shifts (STARS; Rodionov, 2004) was used to analyze trends and step changes in fisheries and climate data, after a “pre-whitening” procedure (Rodionov, 2006) was applied. STARS is written in Visual Basic for Application (VBA) for Microsoft Excel for Windows4. Results of STARS are determined by the cut-off length for proposed regimes (L) and the Huber weight parameters (H), which define the range of departure from the observed mean beyond which observations are considered as outliers. L was set to 20 and H to 1 in this study.
Principal component analysis (PCA), implemented with R package “psych” (Revelle, 2017), were applied to the fisheries catch, OCOM-estimated biomass and RAPG of the seven piscivorous fish to isolate the most important patterns of variations in these time series. In addition, correlation analysis combined with significance tests were also conducted using “psych” to examine relationships between fisheries catch and environmental and fishing variables.
In order to understand how fishing, climatic, and oceanographic conditions have impacted the dynamics of the seven piscivorous fish, we applied the gradient forest method (Ellis et al., 2012). The gradient forest method is built upon the random forest method, inheriting all its functionalities, and extends to multiple response variables, which allows one to quantify the degree of community change along the predictor gradient. The most important feature is that the gradient forest method can capture complex relationships between potentially correlated predictors and multiple response variables by integrating individual random forest analyses over the different response variables (Ellis et al., 2012). Each random forest is a large set of regression trees that partition the response variables into two groups of maximum within-group homogeneity by selecting a split value s for each predictor p. Each tree is fitted using an independent bootstrap sample of data, while a cross-validated estimate of the generalization error is provided by the data that are not selected in the bootstrap sample for a tree (out-of-bag OOB data). The gradient forest method is implemented with two R packages “extendForest” and “gradientForest” (R Core Team, 2018). In this study, using 16 environmental and anthropogenic (i.e., fishing effort) variables as predictors, we ran the gradient forest analysis 1,000 times to obtain the mean and standard deviation (sd) of good-of-fit (R2). The run with the highest R2 was then used to derive all statistics. By quantifying the changing patterns of response variables (i.e., catch and estimated biomass of piscivorous fish) along the gradients of environmental and fishing variables and assessing predictor importance, gradient forest analysis can identify important thresholds in environmental gradients.
Results
Variations in Piscivorous Fishes
Chinese catch of piscivorous fish gently increased from 1955 to 1990 before sharp increases in the 1990s and stabilized around two million metric tons in the past two decades; however, its proportion in total marine catches dropped from 42.4 to 16.8% before 1990 and then slowly climbed up to about 22% (Figure 2A). The engine power of Chinese marine fishing boats increased continuously after 1955 and is now much higher than it was in the early decades of the time series, although it showed slight decline in recent years; the number of fishing boats simultaneously increased after 1955 but declined in the early 2000s (Figure 2B). The estimated CPUE dropped sharply in the 1950s then remained below 50 tons per boat or 80 tons per 100 kw, which indicated over exploitation in the China Seas.
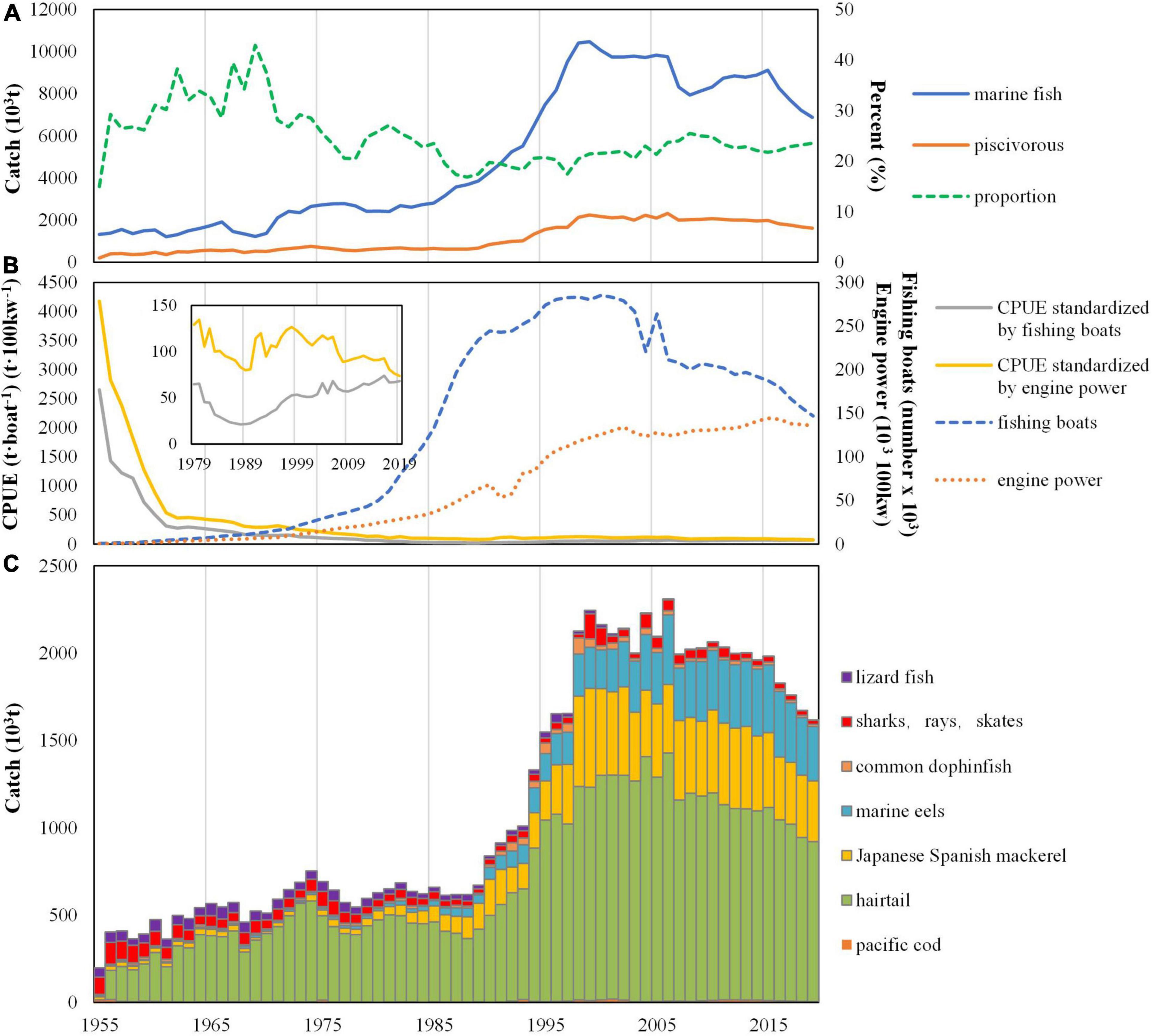
Figure 2. Annual variations in catch of marine fish and piscivorous fish in China for the period of 1955–2016. (A) Trends in catch of marine fish and piscivorous fish as well as proportion of piscivorous fish catch in the total catch of marine fish. (B) Marine fishing boats and their engine power, and CPUE of marine fishes by fishing boats and engine power, respectively. (C) Catch of piscivorous fish.
Among the seven taxa of the piscivorous fish, hairtail, Japanese-Spanish mackerel, and eels contributed the most to the catch (Figure 2C). Catch trends of the seven piscivorous fish generally showed decadal variations (Figure 3). The catch of Pacific cod decreased from the late 1950s to 1990s and then increased but with large fluctuation during the entire time period from 1955 to 2016 (Figure 3A). The catch of hairtail and Japanese-Spanish mackerel showed slight growth from 1955 to 1990 and increased drastically during the 1990s before decline in the 2000s (Figures 3B,D). Marine eel continuously increased after 1955 particularly after 1990 (Figure 3C). The catch of common dolphinfish fluctuated and maintained around 10 thousand tons, reaching about 20 thousand tons during the period of 1990–2003 followed by a sharp decline (Figure 3E). The catch of lizardfish was volatile from 1955 to 1966 but decreased particularly after 1980 while sharks and rays showed a persistent decline after the 1950s with only occasional increase (Figures 3F,G). Previous researches have indicated the declines of several species in the taxon of sharks and rays, contributing to the decline of this taxon. For instance, blackspot shark (Carcharhinus menisorrah) used to be a dominant species of Beibu Gulf in the South China Sea during the 1960s yet declined severely ever since (Sun and Lin, 2004); Ocellate spot skate (Raja porosa) was also a dominant species of the Bohai Sea before 1983, but became a rare species since 1999 (Deng and Jin, 2001).
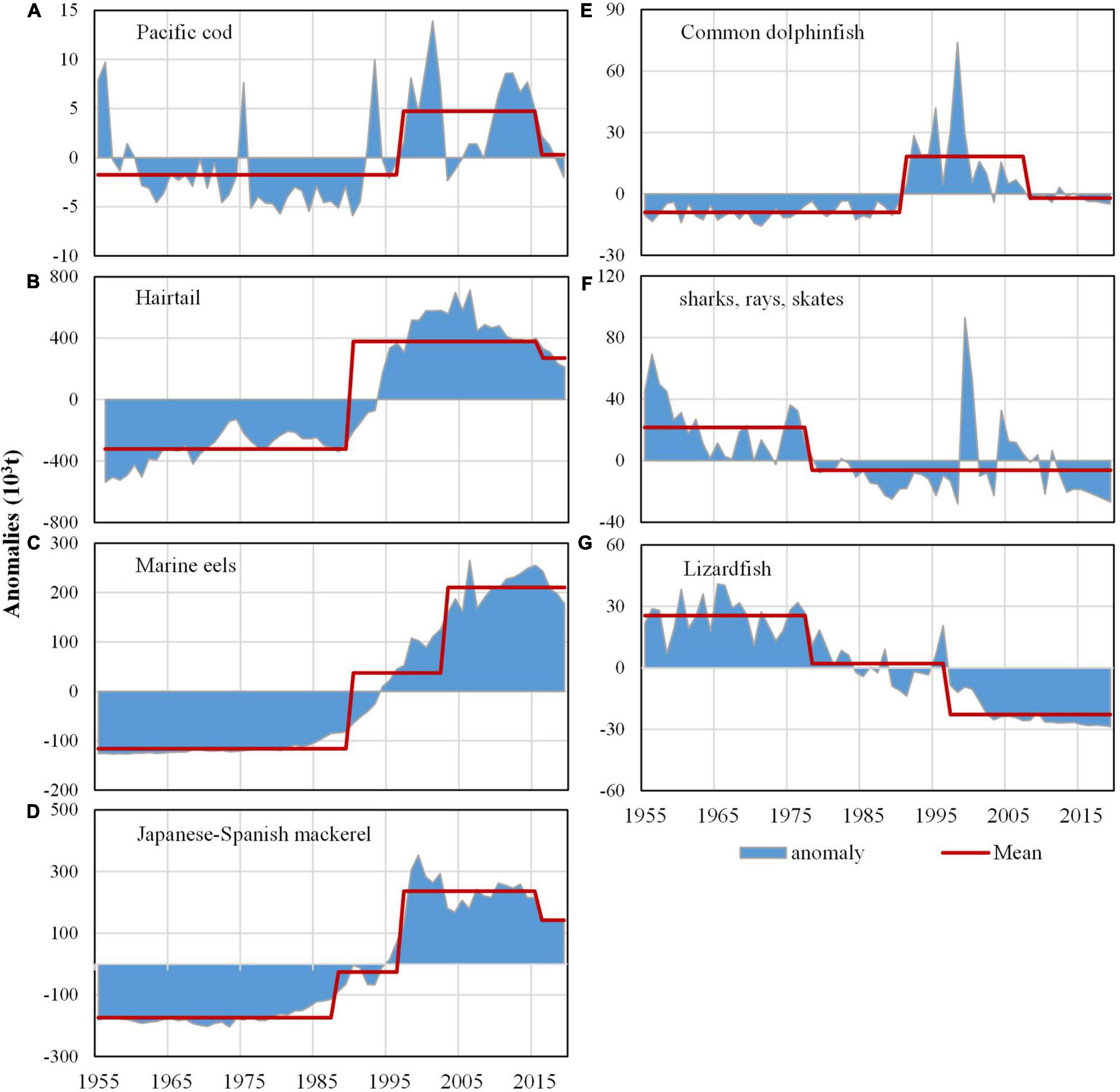
Figure 3. Catch of piscivorous fish in China: (A) Pacific cod, (B) Hairtail, (C) Marine eel, (D) Japanese-Spanish mackerel, (E) Common dolphinfish, (F) Sharks, rays and skates, and (G) Lizardfish. Shaded areas show catch anomalies; red lines represent means of anomalies in different phases that were calculated by STARS.
Step changes within 2015–2018 for catches of piscivorous fish should be treated with caution since they are at the end of time series and would need to be verified with additional data. Thus combining with regime shift detection (Figure 3),four different changing patterns were observed in the catch of piscivorous fish: (1) Hairtail, marine eel, and Japanese-Spanish mackerel showed abrupt changes around the late 1980s with continuous increasing trends, followed by a second step change around 1997/98 for Japanese-Spanish mackerel and 2002/03 for marine eel; (2) the taxa of sharks, rays, skates, and lizardfish kept decreasing with step changes in 1977/78 followed by a second step change around 1996/97 for the latter; (3) Common dolphinfish was relatively stable before an abrupt increase around 1990/91 and a subsequent decrease in 2007/08; and (4) Pacific cod fluctuated wildly with a step upward around 1996/97. To summarize, three main step changes were observed in the catch of piscivorous fish around 1977/78, 1988–90 and 1997/98. PCA results for the catch of the seven piscivorous fish indicate that only the PC1 is meaningful, explaining 63% of variance. Decadal variabilities in PC1 is apparent with step changes around 1985/86, 1997/98 (Figure 4).
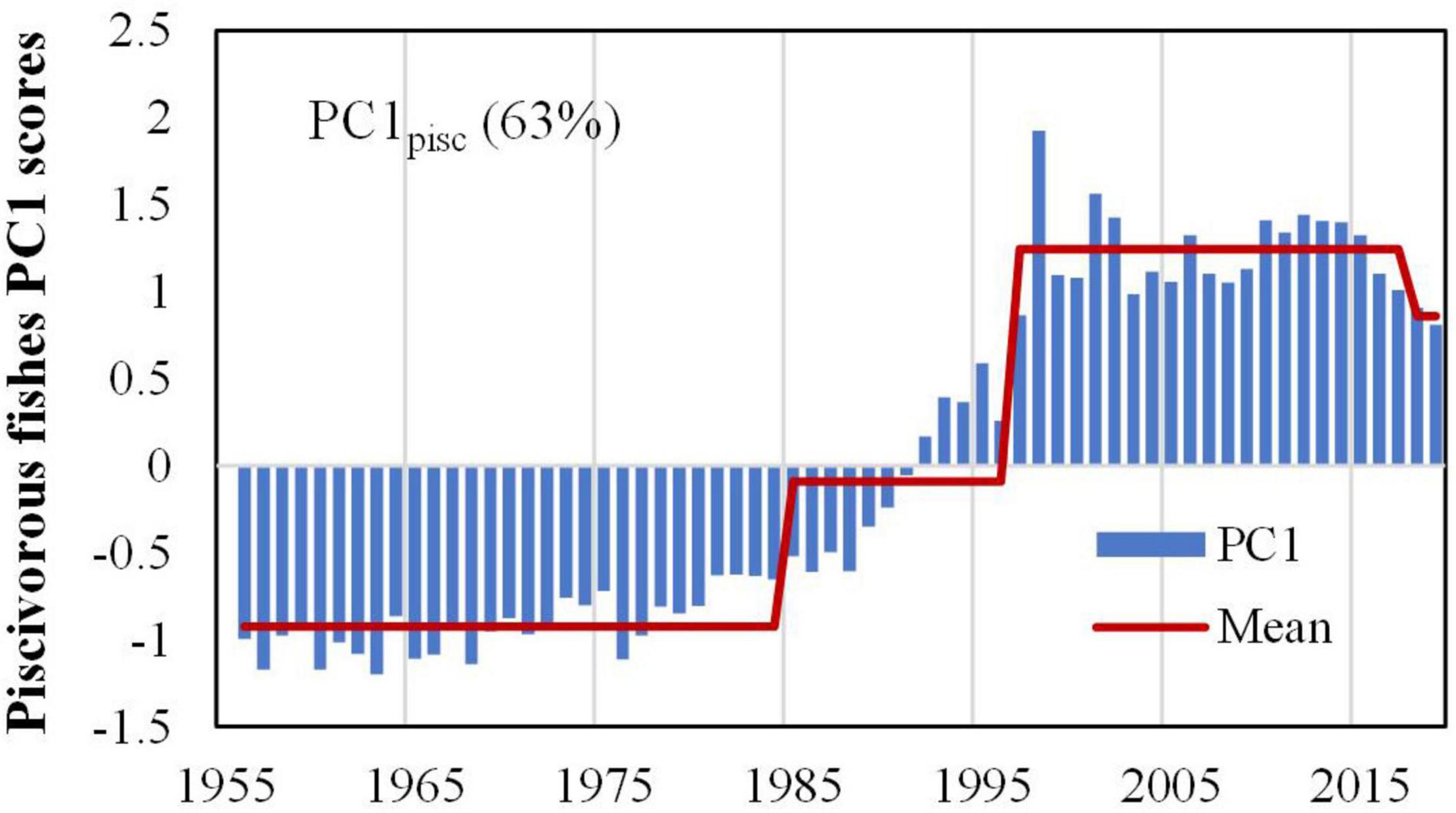
Figure 4. Principal component analysis (PCA) of the catch of the seven piscivorous taxa shows regime shifts in PC1 (explaining 64% of the variations).
The estimated biomass of the piscivorous fish also showed inter-decadal variations with drastic decline during 1990s for most of the taxa (Supplementary Figure 3). Compared with biomass, the RAPG showed similar changing patterns except with an opposite direction. In general, variations in the estimated biomass and RAPG highlight the step change that occurred in the early 1990s. And their PCA results both showed step changes that occurred in 1992/93 (Supplementary Figure 3).
Changes in Biological Characteristics of Piscivorous Fish
Due to lack of long time series of biological data for the seven piscivorous fish, our analyses of life history traits are limited to those that have biological data.
For hairtail in the ECS, age composition showed drastic change from the 1960s to the 1990s (Figure 5A). Prior to the mid-1980s, age-1 was the most dominant; however, its dominancy reduced in the mid-1980s, and age-0 contributed to about 50% of the age composition in recent decades (Figure 5A). Consequently, average age of hairtail in the ECS declined over time. In addition, average snout-vent length also declined with step-like change occurring around 1990/91 (Figure 5B), corresponding to the regime shift around 1990/91 in catch and estimated biomass of hairtail.
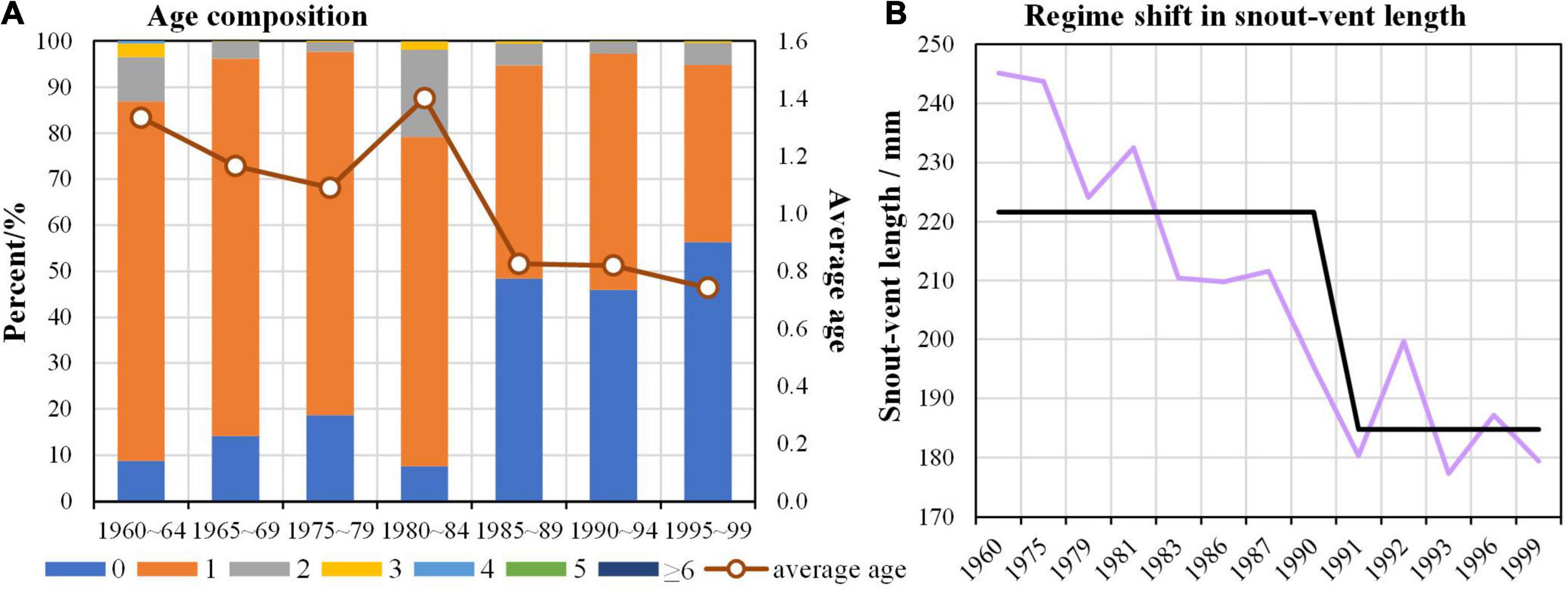
Figure 5. Changes in life history traits of hairtail (Trichiurus japonicus) from the East China Sea: (A) Variations in age composition; (B) Changing trend in snout-vent length with a step change.
Average age of Japanese-Spanish mackerel from the Bohai Sea and the Yellow Sea from 1988 to 1994 showed a generally declining trend, while the proportion of mature individuals at age 1 increased from 25% to over 90% (Figure 6A), indicating earlier sexual maturity. Both minimum fork length and weight at maturity of females and males decreased from 1960 to 2005 with a common step change occurring in 1991 (Figures 6B,C).
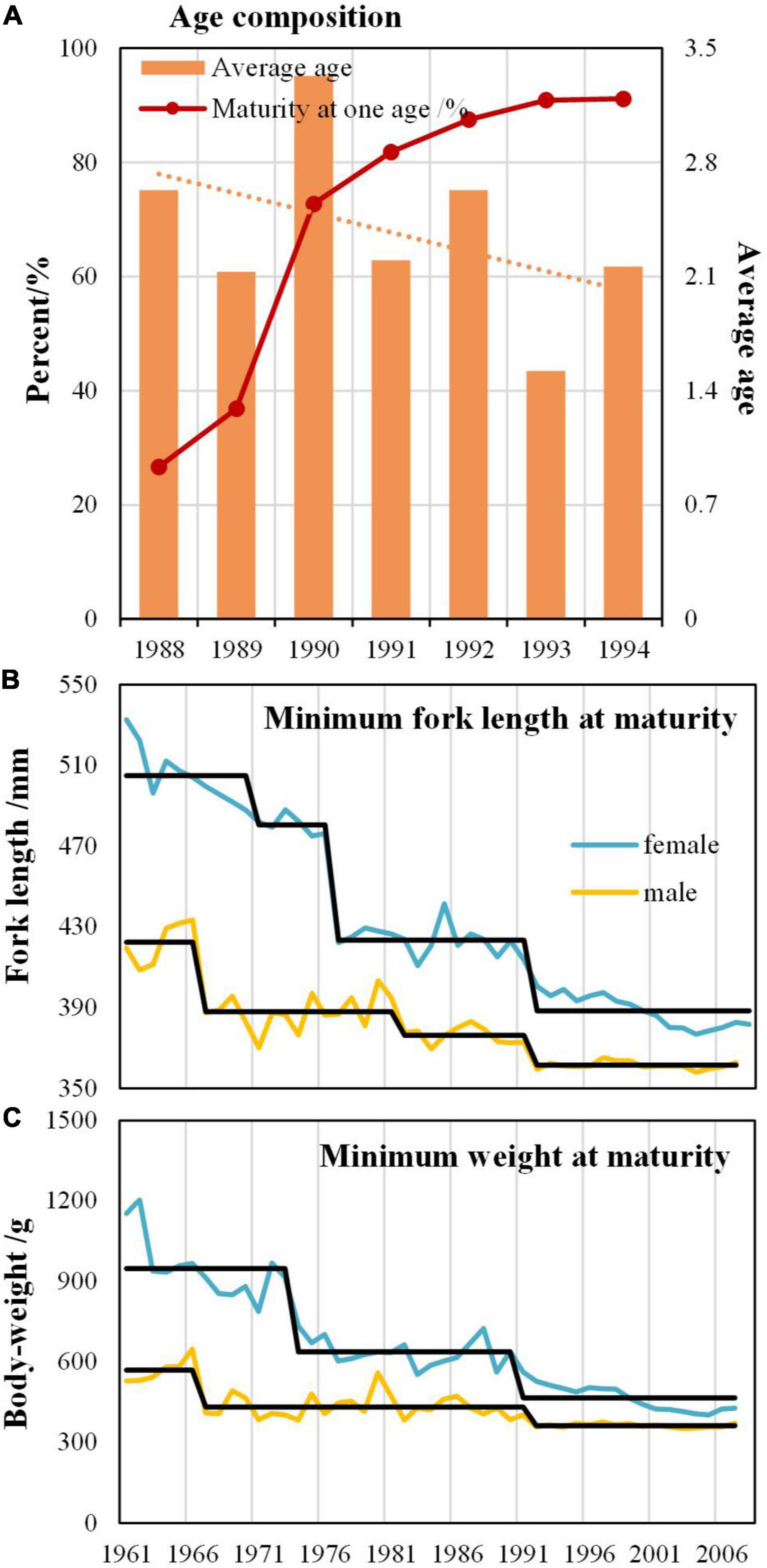
Figure 6. Changes in life history traits of Japanese-Spanish mackerel (Scomberomorus niphonius) from the Bohai Sea and the Yellow Sea: (A) variations in age composition; (B,C) variations in body-size with step changes.
Pacific cod showed another pattern of variations in biological characteristics. Both the range of body length and average body length increased from 1999 to 2009 (Li et al., 2012) after the increase in catch of Pacific cod with step change in 1996/97. And during the same period, estimated biomass of Pacific cod showed a changing trend from dropping at first to rising afterward. For marine eel, catch was dominated by individuals of 250–400 mm long in the 1960s and 1970s, and since the 1990s it has become more dominated by younger fish with snout-vent length less than 250 mm. Moreover, the size at sexual maturity (L50) dropped over 50 mm since the 1980s (Dou, 2014). The lack of biological data for common dolphinfish, lizardfish and sharks and rays in our study regions does not allow us to draw conclusions on their changes in biological characteristics. However, studies from the South China Sea suggest that body length and weight for lizardfish have declined (Supplementary Figure 1), and sharks and rays populations have been depressed (Zhang and Yang, 2005).
Response of Piscivorous Fish to Climate Variability
Step changes in the catch of the seven piscivorous fish corresponded well with those in the regional SST indices (Figures 7A,B). The step change of Pacific cod catch in 1997/98 (Figure 7A) preceded the step change of PC1 of summer SST by 3 years in both the YS and ECS in 1994/95 (Figure 7B and Supplementary Figures 2A,B). The step change of hairtail catch in 1990/91 (Figure 7A) preceded the step change of PC1 of winter SST by 4 years in both the YS and ECS in 1986/87 (Figure 7B and Supplementary Figures 2C,D). The time lags between step changes of fisheries catch and those of SST were also evident for Japanese-Spanish mackerel, marine eel and common dolphinfish. Step changes in SSS largely occurred around 1998/99 in both summer and winter (Figure 7B and Supplementary Figures 2E–H); however, they did not appear to match the variations in piscivorous fish catch (Figure 7A).
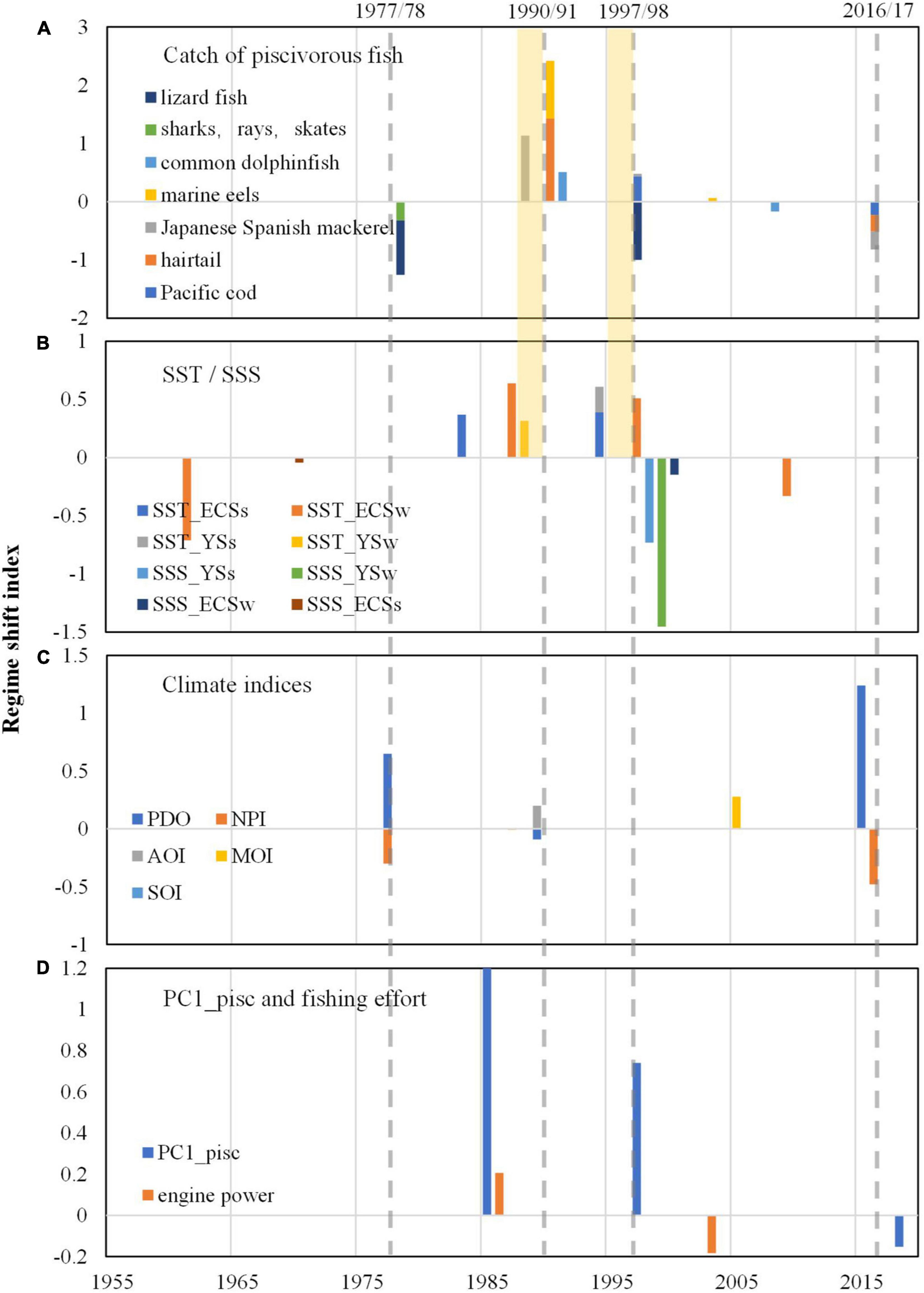
Figure 7. Regime shift indices calculated by STARS: (A) for catch of piscivorous fishes; (B) for PC1 of SST (sea surface temperature) and SSS (sea surface salinity) indices; (C) for climatic indices. Pink dotted bars show gaps between catch and SST (e.g., winter and summer SSTs for the ECS are abbreviated as SST_ECSw and SST_ECSs, and so on) were the most important predictors with the highest mean importance, followed by SSS_YSw, SSS_YSs, SST_YSw and SST_YS.); (D) for PC1 of piscivorous catch and Chinese total marine fishing engine power (105 kw). Note that step changes during the period of 2016–2019 for piscivorous catch, PC1 of piscivorous catch and climate indices should be treated with cautions since they are at the end of time series and would need to be verified with additional data.
Compared to the SST indices, climatic indices had step changes that were less correlated with those in piscivorous fish catch (Figures 7A,C). However, the step change around 1977/78 in the catches of sharks, rays, and lizardfish corresponded well to the regime shifts of PDO and NPI, and the step changes around 1990/91 in hairtail, marine eel, and common dolphinfish corresponded well to that of PDO and AOI.
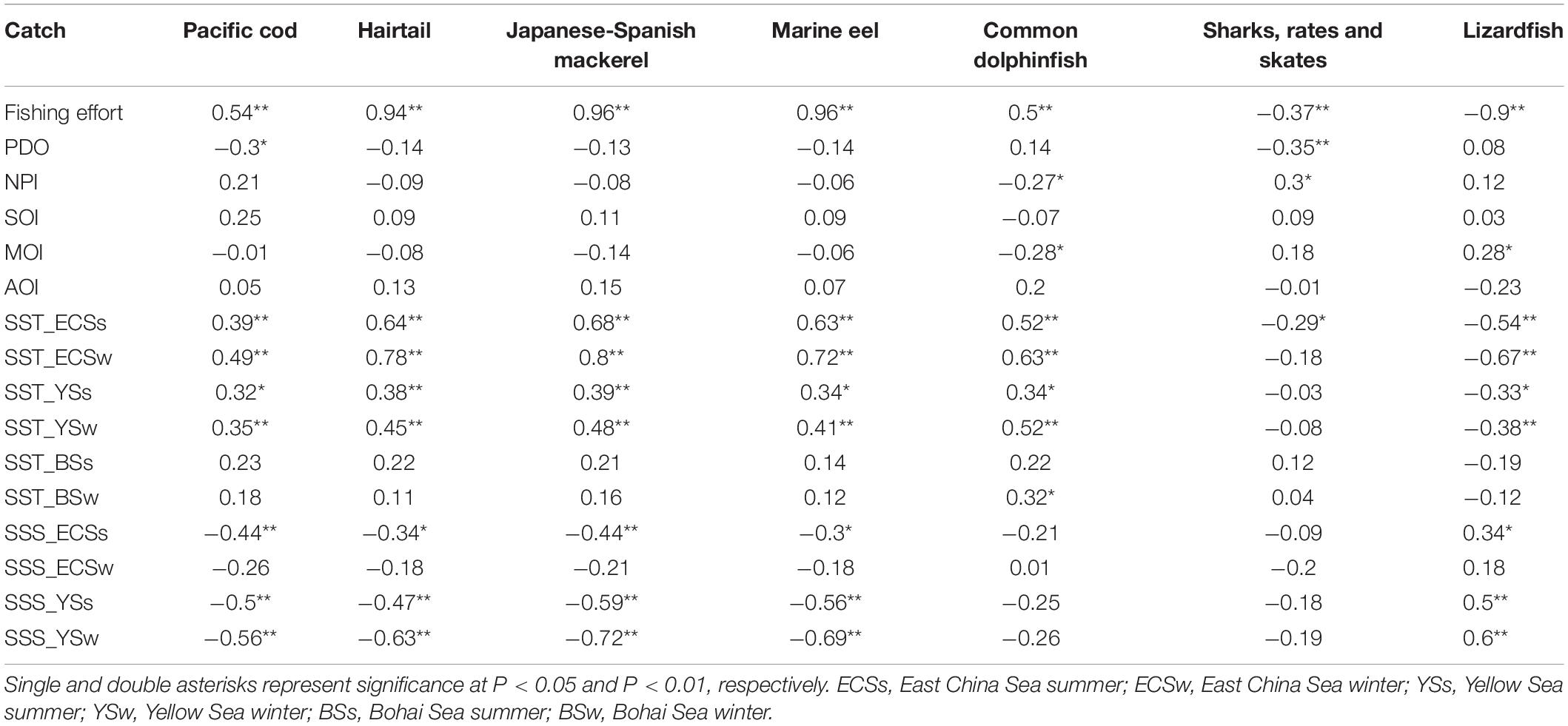
Correlation analyses between catch and environmental and fishing variables indicated that except for sharks and rays, catches of piscivorous fish were significantly correlated with SSTs in the ECS and YS, and the correlation was positive with the exception of lizardfish (Table 2). On the other hand, except for common dolphinfish, sharks and rays, other piscivorous fish also had significant correlation with SSSs, and it is worth noting that the signs (i.e., positive and negative) of the correlations were totally opposite to those in SSTs. Catches of sharks and rays were not significantly correlated with SSTs or SSSs; however, they were significantly correlated with PDO and NPI (Table 2).
Gradient Forest Analysis for Catch Data
Gradient forest analyses were carried out to explore the responses of fisheries catch, estimated biomass and RAPG of the seven piscivorous fish to fishing effort (i.e., total engine power of Chinese marine fishing boats), oceanographic data (i.e., SST and SSS) and climatic indices (i.e., PDO, SOI, AOI, and MOI). We only present the results for fisheries catch as results for estimated biomass and RAPG were rather similar.
In order to understand how the long-term fishing pressure might have impacted the gradient forest analysis results for different piscivorous fish, we conducted analyses for two scenarios: with and without fishing effort being included as a predictor. The differences between the two scenarios in terms of their performance (goodness-of-fit R2) varied among the different piscivorous fish (Figure 8A). The R2 values had the smallest difference between the two scenarios for catch of Pacific cod, indicating the fishing pressure had the least effect on this species. The R2 values for catches of Japanese-Spanish mackerel, marine eels, hairtail were greater than 0.6 even when fishing effort was not included as a predictor, suggesting that these three taxa were well explained by the predictors and they were more responsive to climate variability than to fishing effort. On the other hand, the R2 values for catches of lizardfish, common dolphinfish, and Pacific cod were generally lower than 0.5 when fishing effort was not included as a predictor. Sharks and rays showed a near-zero R2 value when fishing effort was not included, suggesting that the temporal dynamics of this piscivorous taxon cannot be explained by any environmental variables included in the analyses.
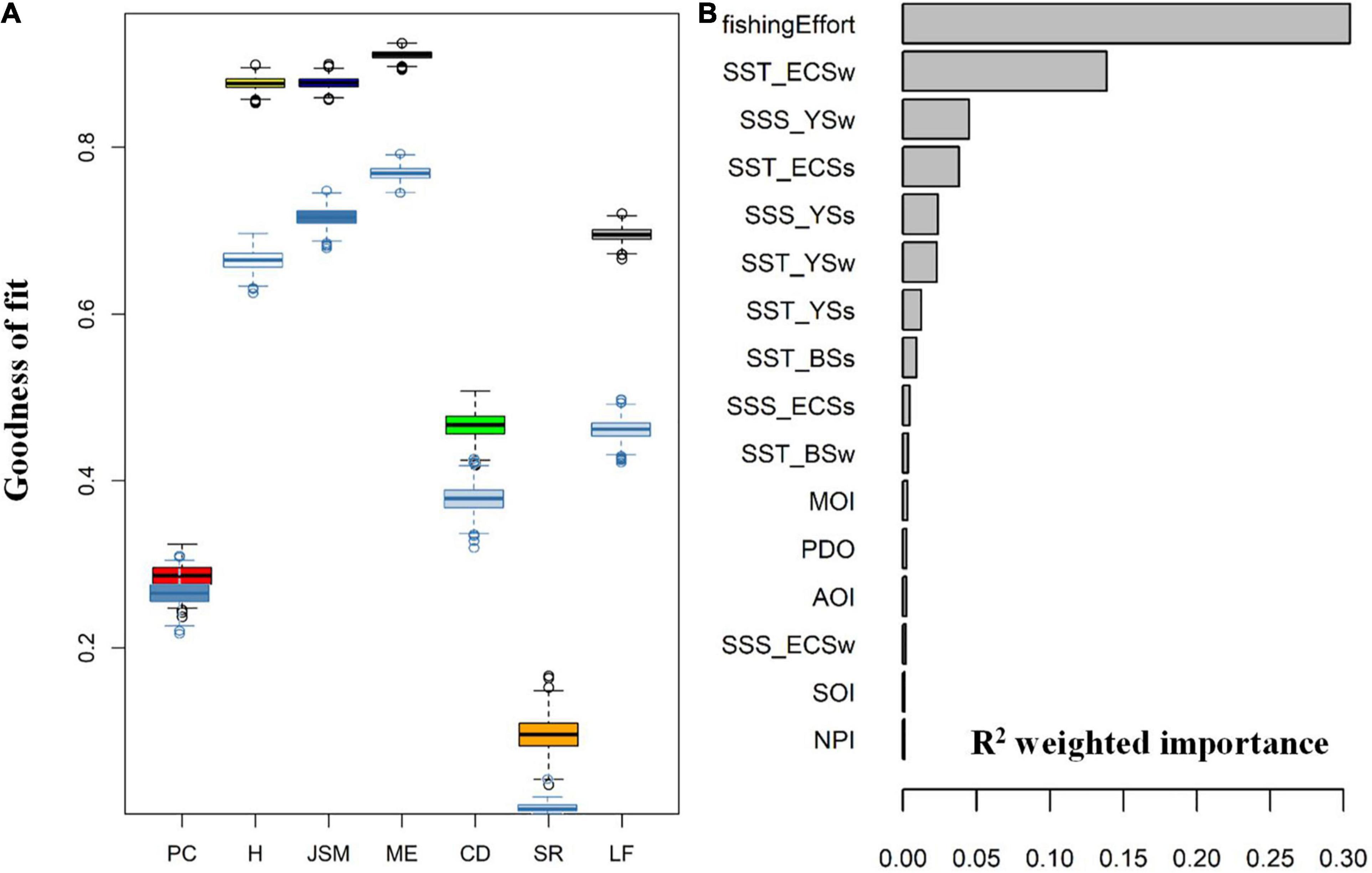
Figure 8. (A) Goodness-of-fit R2 of the gradient forest analysis for seven Chinese piscivorous fish with PC, H, ME, JSM, CD, SR, and LF representing Pacific cod, hairtail, marine eel, Japanese-Spanish mackerel, common dolphinfish, sharks and ray, and lizardfish, respectively, whereas GF results with no fishing effort were showed in nattier blue; (B) the mean importance of each variable weighted by species R2, including fishing effort, various climatic indices (PDO: Pacific Decadal Oscillation, NPI: North Pacific Index, AOI: Arctic Oscillation Index, SOI: Southern Oscillation Index, and MOI: Asian Monsoon Index) and sea surface temperature (SST) data in different seas and seasons (BSs, Bohai Sea summer; BSw, Bohai Sea winter; YSs, Yellow Sea summer; YSw, Yellow Sea winter; ECSs, East China Sea summer; ECSw, East China Sea winter).
Considering the overall impacts on the seven piscivorous fish, the first six most important predictors include fishing effort, winter SSTs for the ECS (i.e., SST_ECSw), SSS_YSw, SST_ECSs, SSS_YSs, SST_YSw, and SST_YSs (Figure 8B). The predictor fishing effort, ranked first in its R2-weighted importance, was most influential in determining the dynamics of the piscivorous fish community. However, the importance of SST_ECSw was also undeniably high, indicating that the piscivorous fish community was also subjected to region-scale environmental change. In contrast, all the climatic indices had very low R2-weighted importance.
The responses of the seven piscivorous fish to fishing effort, the most important predictor, displayed different thresholds along the gradient of fishing effort (Figure 9A). The catches of lizardfish and sharks and rays had the lowest threshold response around 20 (∗105 kW), indicating that these two piscivorous taxa were most sensitive to fishing pressure. The catches of hairtail and marine eel had strong threshold responses when fishing effort was around 80 (∗105 kW). Compared with all other piscivorous fish, Japanese-Spanish mackerel appeared to be least sensitive to fishing pressure with its catch shifting primarily in response to high fishing effort around 100 (∗105 kW). In responses to SST_ECSw, the second most important predictor, major catch shifts occurred around 16.5°C for Japanese-Spanish mackerel, marine eel, hairtail, lizardfish, and common dolphinfish while that for Pacific cod occurred around 17°C (Figure 9B). The catches of Japanese-Spanish mackerel, marine eel, hairtail also responded more strongly than other piscivorous taxa to SST_ECSs with a split value around 28.0°C (Figure 9D). Important split values occurred around 31.5–31.6‰ and 29.4‰ along the gradients of SSS_YSw and SSS_YSs, respectively, for most of the piscivorous taxa with marine eel, Japanese-Spanish mackerel, and hairtail showing the highest responsiveness (Figures 9C,E). As predictors (e.g., SSS_YSs, SST_YSw) decreased in their importance, the catches of the piscivorous fish became less synchronized in their responses to the predictors (Figures 9E,F).
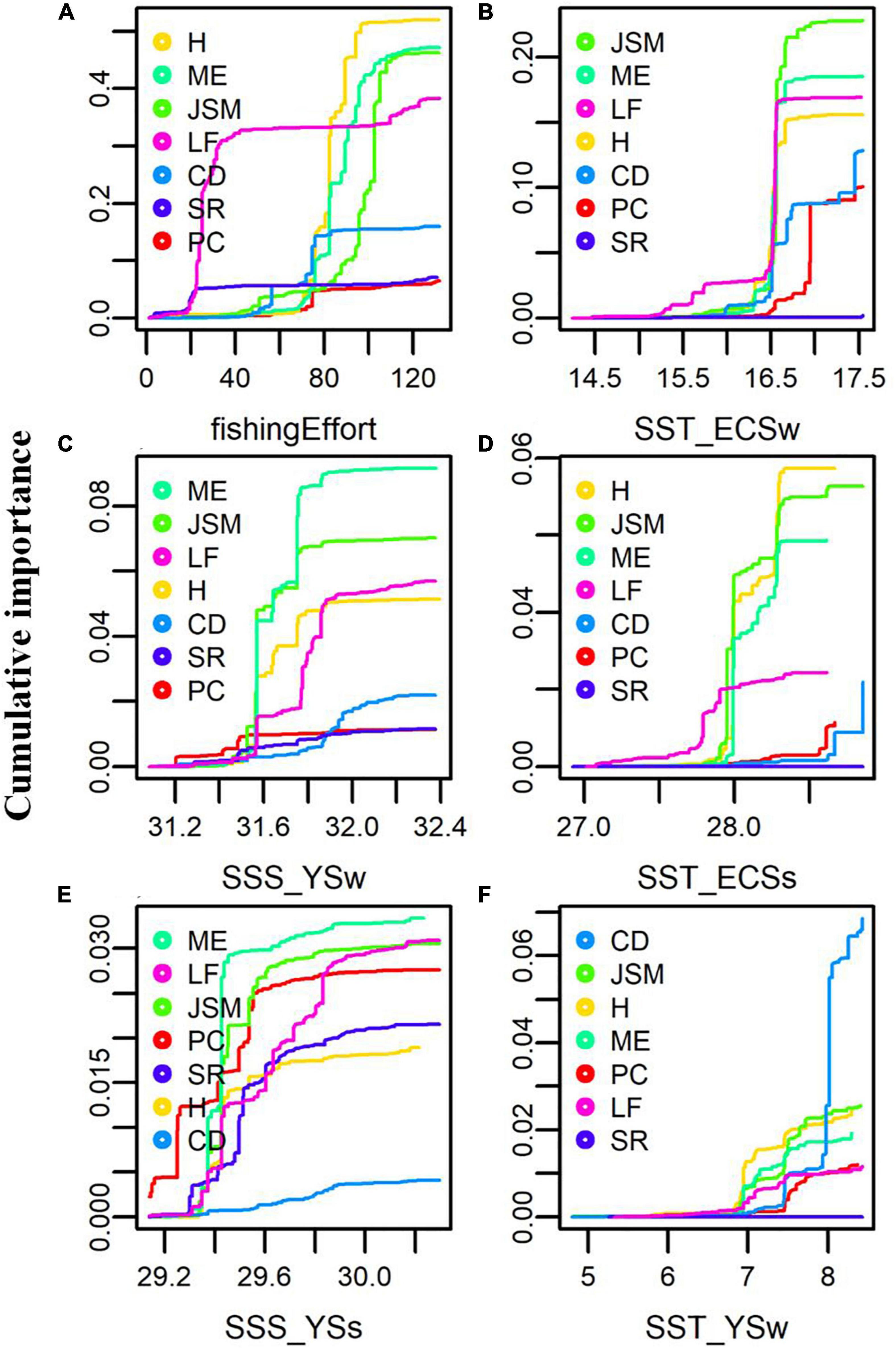
Figure 9. Cumulative importance of piscivorous fish catch in response to the first six important predictor variables including (A) fishing effort, (B) sea surface temperature (SST) in East China Sea winter (ECSw), (C) sea surface salinity (SSS) in Yellow Sea winter (YSw), (D) SST in East China Sea summer (ECSs), (E) SSS in Yellow Sea summer (YSs), and (F) SST in Yellow Sea winter (YSw). PC, H, ME, JSM, CD, SR, and LF represent Pacific cod, hairtail, marine eel, Japanese-Spanish mackerel, common dolphinfish, sharks and ray, and lizardfish, respectively.
In order to tease apart the potentially confounding effects of regime shift in ocean conditions and big shift in fishing effort around 1990, we divided the time series data of piscivorous catches into two sets with the year 1990 as the boundary to conduct gradient forest analyses. Prior to 1990, the R2 values for all piscivorous fish except for Pacific cod and common dolphinfish were high around 0.5 when fishing effort was included as a predictor (Supplementary Figure 4A and Supplementary Table 3). However, the R2 values reduced to very low levels or to zero when fishing effort was excluded, indicating that fishing pressure afflicted the greatest impacts on the dynamics of these five piscivorous fish in this earlier period. For the latter period (1990–2019), the R2 differences between the two scenarios (with and without fishing effort) were much smaller (Supplementary Figure 4B and Supplementary Table 3), indicating that environmental variability started to play a greater role in shaping the dynamics of the piscivorous fish in the last two decades.
Discussion
Decadal Variation Patterns in Piscivorous Fish and the Effect of Climate Regime Shift
The analyses of piscivorous fish in this study were based on the catch data from the Chinese Fishery Statistics. The quality of these catch data has been questioned (Szuwalski et al., 2017). The Sea Around Us project intended to mitigate the over-inflation in Chinese catch to benefit aggregated, long-termed and global- or regional-scale research (Pauly and Zeller, 2016). Our comparisons between with the catch data from Chinese Fishery Statistics and those reconstructed by the Sea Around Us reveal that these two data sources show similar variation trends, although the overall catch levels are lower from the latter. Both catch data correspond well with changes in fishing effort that increased rapidly from the 1980s to 1990s with the expansion of fishing grounds, and remained stable ever since. Because the purposes of this research are to investigate temporal variabilities of piscivorous fish at community level and to understand the impacts of fishing and oceanographic/climatic changes on the piscivorous fish at community, we do not consider the potential over-inflation of our catch data from the Chinese Fishery Statistics a problem.
Three major step changes around the late 1970s, early 1990s, and late 1990s were identified in the catch of the seven piscivorous fish (Figure 7). During the period of 1955 to 2019, the first downward step change was detected in the catch and biomass of lizardfish, sharks and rays around 1977/78, corresponding well to the regime shift in PDO and NPI. Despite the hysteresis observed between catches and SSTs, step changes in the PC1 of piscivorous fish catch occurred in 1985/86 and 1996/97, the latter one of which corresponded well to the step changes in SST indices. Step changes around the late 1980s and 1990s have also been identified in small pelagic fishes and cephalopods in the China Seas (Pang et al., 2018; Ma et al., 2019). Previous studies have clearly documented the relationship between climate regime shifts and abrupt changes in fisheries catch in the northwestern Pacific (Tian et al., 2004, 2008; Jung et al., 2017). Accordingly, these three step changes detected in the piscivorous fish in the China Seas were apparently related to the well documented climatic regime shifts that occurred in the North Pacific in 1977, 1989, and 1998 (Hare and Mantua, 2000; Kang et al., 2012).
All warm-water taxa, except for lizardfish, sharks and rays, showed increasing trends in fisheries catch with a main step change around 1990/91 under continuous warming trend of seawater. The increase of warm-water taxa was also observed in the East Asian Marginal Seas, following the increase of zooplankton biomass particularly that of copepod that was caused by the climate warm regime in the 1980s (Jung et al., 2017). Results of gradient forest analysis also indicated that warm-water taxa (except for sharks and rays) were more responsive to climate variability.
Although relevant studies show that increasing sea temperature had negative impact on larval cod survival (Lehodey et al., 2006), Pacific cod, the only cold-water taxon in our study, still increased with step change around 1993/94 and 1997/98 in estimated biomass and catch, respectively. Even both the average body-length and relative population density distinctly increased from 1999 to 2009, which indicated a completely different pattern from other piscivorous fish. Further research showed that in contrast to the warming trend from 1950 to 2017, there was a surface cooling associated with climate regime shift in 1998 (Kim et al., 2018). The Yellow Sea Cold Water Mass, the essential habitat for Pacific cod, revealed a cold event that began in 1996 after a warm event during 1990–1995 and extended to the coast to cover the entire YS trough (Park et al., 2011). This alternation from warm to cold water in the Yellow Sea probably resulted in the step change of Pacific cod around 1997/98 and the subsequent changes in biological characteristics. Generally, the diversity of changing patterns indicated that the response of piscivorous fishes to climate change varied by taxa and regions (Jung et al., 2017).
Impact of Fishing on Piscivorous Fish in the China Seas
China has implemented a series of policies to control the fishing intensity, including “Double-Control” system (controlling the number and engine power of fishing vessels) since 1987 and summer fishing moratorium since 1995. But available biological data from the China Seas indicated significant changes, happening particularly around 1990, in the life history traits of most piscivorous fish such as reduced average body sizes and truncated age compositions, implying the strong effect of fishing, which echoes the increasing concern over the effects of fishing on exploited stocks (Planque et al., 2010; Tu et al., 2018).
Compared to environmental variables, fishing intensity showed greatest impacts on variations in piscivorous fish (Figure 8B). In addition, step changes of Chinese fishing effort (total engine power) occurred in 1985/86 and 2002/03 (Figure 7D), of which the former one was consistent with that of PC1_pisc occurred in 1985/86. These step changes were close to that of piscivorous catches and estimated biomass detected in 1990/91 and 1992/93, respectively, indicating that the rapid growth of fishing effort from 1985 may lead to the increasing step change of piscivorous catches around the late 1980s and the early 1990s and subsequently decrease of estimated biomass. With continuous increase in fishing intensity and shrinking mesh size of fishing gear during the recent five decades, hairtail, marine eel and Japanese-Spanish mackerel decreased in estimated biomass and CPUE, as well as body size and age at maturity (Figures 5, 6). Although smaller meshes would also lead to changes in mean sizes of fisheries catch, exploited fish have already become smaller in size and younger in age. The average age of Japanese-Spanish mackerel, for instance, decreased from 2.83 to 1.98, and the dominant age group changed from ages 2 and 3 to ages 1 and 2, and the average fork length and weight decreased from 583 mm and 1,507 g to 521 mm and 1,091 g, respectively; after 1990, due to the increase of mesh size of drift gill net and the use of sparse trawl, the average age, fork length and body weight increased to 2.65 years old, 574 mm and 1,359 g, respectively, which are obviously lower than those of unexploited populations, and the population was still dominated by 1- and 2-year-old fish (Sun, 2009). It displayed the situation that landings remained high due to high fishing effort whereas stock biomass decreased, which corresponded typically to overfishing (Travers et al., 2010) resulting in depletion in fishery resources. This situation was even worse with lizardfish, sharks and rays with catch, estimated biomass and life history trait continuously declining in the past few decades (Figure 3 and Supplementary Figures 1, 3).
Warming trends in marine ecosystems strongly influence biological processes such as growth, reproduction, phenology, distribution, recruitment, and mortality (Rochet et al., 2010). A warm year may lead to a strong year class and therefore affect the size structure of fish stock (Pekcan-Hekim et al., 2011), whereas the effect of temperature on the asymptotic body size may persist for several years (Tu et al., 2018). Decadal variations in body-size occurred around 1975/76 and 1990/91 for Japanese-Spanish mackerel and 1991/92 for hairtail, suggesting that the changes in the life history traits as well as abundance corresponded to the climatic regime shifts in the North Pacific. However, the trend of reduced average body sizes and truncated age compositions in the catch occurred concurrently with sharp decrease in CPUEs of Chinese piscivorous fish from 1955 to the 1970s; for example, step changes of Chinese fishing effort, catch, and body-size of Japanese-Spanish mackerel were detected in 1985, 1988, and 1990, suggesting that changes in life history traits of piscivorous fish were largely caused by continuous high fishing pressure rather than climate regime shifts.
Pacific cod, however, was an exception. The catch and biological data both increased since the 1990s. And Pacific cod have lower economic values compared with other large fish such as Japanese-Spanish mackerel. Therefore, compared with the impact of fishing, environment effects caused by climate change (e.g., recent surface cooling in the YS) contributed relatively more to the variations in Pacific cod, particularly considering that Pacific cod inhabit in the deeper Yellow Sea Cold Water Mass.
Joint Effects of Climate and Fishing
To evaluate the impacts of fishing and environmental variability on fish communities, we need to integrate trends in multiple metrics that respond differently to major environmental pressures (Rochet et al., 2010). Climate indices and fishing effort included in our study were significantly correlated with catch of piscivorous fishes in the China Seas. Although fishing effort was correlated with SSTs in the East China Sea and Yellow Sea (Supplementary Table 2), due to the characteristics of gradient forest analysis, the results based on this method were not affected, which indicated that variations in piscivorous fishes in the China Seas were affected by both climate change and fishing.
Although the impacts of fishing activities on piscivorous fish in the China Seas were much higher (Figure 8B), those of climate change were also significant and cannot be ignored. Some studies suggest that responses of fish communities to climate change are influenced by intense fishing (Pauly et al., 1998; Myers and Worm, 2005), since the responses of populations to environmental effects vary as a function of population characteristics which are subject to change due to fishing, resulting in altered responses to climate change consequently (Perry et al., 2010; Planque et al., 2010). Similar situations were also observed in this study. Taking hairtail from the ECS as an example, with general downward trend linked to high fishing, both the average age and mean snout-vent length increased slightly during the period of 1980–1984 and 1990–1994 (Figure 5), which also well corresponded to climate regime shifts in 1977 and 1989. Such changes were also found in Japanese-Spanish mackerel. On the other hand, step changes of PC1_pisc (PC1 of piscivorous catch) in 1985/86 corresponded well to that of Chinese fishing effort, and both of them were close to step changes of piscivorous catches and climate indices with five and 3-year intervals, respectively. But step changes around 1997/98 were only related to that of climate indices. According to the results of gradient forest analyses, it can be considered that Chinese fishing effort had a greater impact on variations in hairtail, marine eels, sharks and rays, and lizardfish and was mainly concentrated in the period of rapid increase of fishing effort, while the impact of climate change gradually strengthened since the fishing effort was stable and even decreased after 1990. Thus it is suggested that step changes in the late 1980s and early 1990s were more likely to be caused by the joint effects of climate change and increasing fishing pressure, while step changes in 1997/98 were more responsive to climate impact.
In addition, mean trophic level of fishery landings (MTL) decreased before 1990 (Ding et al., 2016, 2017), a phenomenon known as “fishing down marine food webs” (Pauly et al., 1998). However, MTL increased after the late 1980s despite increased fishing pressure (Ding et al., 2017), showing an opposite trend, i.e., MTL should be declining under continuous high fishing pressure (Travers et al., 2010), Such phenomena are likely associated with the large-scale regime shift that occurred around 1989 in the North Pacific. And the proportion of piscivorous fishes in the total catch of marine fish declined while small pelagic fish in the China Seas relatively increased (Supplementary Figure 5), which was consistent with the wasp-waist theory (Cury et al., 2003), i.e., the decline caused by overfishing would propagate up and down the food web and specifically predators would overall decrease in abundance.
Generally, variations of piscivorous fish in the China Seas were related to climate change and fishing effort. And the differences of the performance values between the two scenarios with/without fishing effort (Figure 8A) indicated that the four changing patterns of these different taxa can be summarized as follows: (1) the increase of hairtail, marine eels and Japanese-Spanish mackerel with decadal variations were affected by the interaction of climate change and fishing intensity, (2) the fluctuations of Pacific cod and common dolphinfish were dominated by climate variabilities, and (3) lizardfish, sharks and rays, as early-exploited economic fishes, kept decreasing since the late 1950s, which may result from the persistent fishing pressure.
Implication for Fisheries Managements of Piscivorous Fish
The OCOM is useful to estimate management quantities across a range of stocks simultaneously when only catch data are available and when the average or ensemble statistics become more important (Zhou et al., 2018). Recently, biomasses of some piscivorous fish have been estimated by using the Monte Carlo method CMSY (catch-maximum sustainable yield) (Liang et al., 2020; Zhai et al., 2020). Comparisons between our biomass estimates and those based on CMSY showed similar variation patterns. And combined with the changes of biological characteristics, it is clear that the depletion of fishery resources caused by overfishing have already occurred in the China Seas.
A great challenge for modern fisheries management is to understand multiple sources of variability and their interactions, and take them into account. The traditional goal has been to distinguish between impacts from fishing and those from climate change (Hsieh et al., 2006). In many cases this goal has not been easy to reach as significant changes in exploited marine systems are driven by the interactions between climate change and fishing. By separating the time-series data into two sets, each of which was subjected to two scenarios of gradient forest analyses (with and without fishing effort as a predictor), we were able to demonstrate that fishing pressure had larger impacts on piscivorous fish in the China Seas before 1990. In the last two decades, environmental variability started to play a greater role in shaping the dynamics of the piscivorous fish in the China Seas. However, the continued high fishing intensity in the same period may have exacerbated the impacts from climate change. By reducing fishing effort, the stocks might be likely to recover even under the current environmental conditions.
At present, marine fisheries management in China focuses on controlling fishing intensity, which mainly consists of fishing moratorium system and total allowable catch (TAC) system (Mu et al., 2007), but neglects the impacts of climate change and selective fishing. In addition, although the vast majority of fishery activities ceased during annual summer fishing moratorium from May to August, the utility of this management measure may vary among different species due to the discrepancies of their life history traits. Thus to form a more targeted fishery management system, different management strategies should also be developed for distinct biological groups or even species with different biological characteristics. In addition, climate change has already caused a series of physical environment changes in oceans all over the world (Bindoff et al., 2019; Collins et al., 2019). Therefore, in addition to timely and appropriate reduction of fishing pressure when fish productivity declines, fisheries management for piscivorous fish in the China Seas should take into account the effect of climate variability. In particular, fisheries management needs to be flexible and adaptive to changing ocean and ecosystem conditions, and it is important to identify species-specific response to climate variability and to implement corresponding fisheries management.
For piscivorous fish in the China Seas, perceptible recession becomes increasingly serious under the circumstance of high fishing pressure. Our results indicated that fishing pressure and climate regime shifts had great impacts on variations of large marine predators in the China Seas while small pelagic fish in the same region were largely affected by climate change (Ma et al., 2019). The impact of fishing pressure can be different on the same species under different climate regimes, which can be intensified by unfavorable climatic regimes (Tian, 2009). Piscivorous fish in this study are targeted by different fisheries including gill net, purse seine and trawl in the China Seas; they have different life history traits, and showed different responses to climate variability and fishing pressure. Under the current fisheries management system without regular fisheries monitoring and stock assessment, it is almost impossible to implement a species-specific management strategy for piscivorous fish in the China Seas. Nevertheless, moving toward ecosystem-based fisheries management, which considers the response patterns to climate variability and fishing at fish assemblage level such as piscivorous fish, can assist the current single-species-based fisheries management. We also advocate precautionary fishery practices with the effects of climate change on fish being explicitly taken into account.
Conclusion
Catch of piscivorous fish in the China Seas showed increasing trends since the 1990s along with the total marine fish catch. Time series of catch, estimated biomass and population growth ratio for piscivorous fishes showed evident decadal variation pattern with three step changes around the late 1970, early 1990s, and late 1990s. Although the step change around the early 1990s was more affected by increasing fishing intensity, all step changes corresponded well to climate regime shifts in the North Pacific. With the exception of Pacific cod, the intense fishing has also reduced average body sizes and truncated age compositions in the catch of piscivorous fish. Both climate change and fishing have impacted piscivorous fish in the China Seas. Specifically, fishing pressure had larger impacts on piscivorous fish in the China Seas before 1990. In the last two decades, environmental variability imposed a greater impact on the piscivorous fish, which may have been exacerbated by the continued high fishing intensity. It is likely that the stocks might have recovered even under the current environmental conditions had fishing intensity been reduced. Therefore, fisheries management for piscivorous fish in the China Seas should simultaneously take into account the effects of fishing and climate variability.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
DL, YT, and ZY conceived of the study. SM, DL, JL, and KL conducted the data compilation and analysis. CF, SZ, and PS provided guidance in the methods. DL, YT, and CF wrote and revised the manuscript. YT, JL, PS, and ZY obtained funding for the study. All authors contributed to the article and approved the submitted version.
Funding
This work was partially supported by the National Natural Science Foundation of China (NSFC) (Grant Nos. 41930534, 41861134037, and 41876177).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful to Yoshiro Watanabe, Professor of University of Tokyo, for his meaningful advice in improving the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.581952/full#supplementary-material
Footnotes
- ^ http://www.seaaroundus.org/
- ^ https://www.metoffice.gov.uk/hadobs/hadisst/index.html
- ^ https://climatedataguide.ucar.edu/climate-data/soda-simple-ocean-data-assimilation
- ^ http://www.beringclimate.noaa.gov/regimes/index.html
References
An, Y., Park, J., Kim, H., and Baeck, G. (2012). Feeding habits of daggertooth pike conger Muraenesox cinereus in the coastal water off Goseong, Korea. Kor. J. Fish. Aquat. Sci. 45, 76–81. doi: 10.5657/KFAS.2012.0076
Belkin, I. M. (2009). Rapid warming of large marine ecosystems. Prog. Oceanogr. 81, 207–213. doi: 10.1016/j.pocean.2009.04.011
Bennett, S., Wernberg, T., Harvey, E. S., Santana-Garcon, J., and Saunders, B. J. (2015). Tropical herbivores provide resilience to a climate-mediated phase shift on temperate reefs. Ecol. Lett. 18, 714–723. doi: 10.1111/ele.12450
Bindoff, N. L., Cheung, W., Kairo, J. G., Aristegui, J., and Whalen, C. (2019). “Changing ocean, marine ecosystems, and dependent communities (09 SROCC Ch05 FINAL-1),” in IPCC Special Report on the Ocean and Cryosphere in a Changing Climate, 2019-09-24, eds H. O. Pörtner, D. C. R. Masson-Delmotte, V. Zhai, P. Tignor, M. Poloczanska, E. Mintenbeck, et al. (Geneva: IPCC).
Butchart, S. H. M., Walpole, M., Collen, B., van Strien, A., Scharlemann, J. P. W., Almond, R. E. A., et al. (2010). Global biodiversity: indicators of recent declines. Science 328, 1164–1168. doi: 10.1126/science.1187512
Chen, Z., Qiu, Y., Xu, S., and Huang, Z. (2012). Evolution of biological characteristics of Sauida undosquamis (Richardson) in the Beibu Gulf, South China Sea. J. Fish. Sci. Chin. 19, 321–328. doi: 10.3724/SP.J.1118.2012.00321
Christensen, V., Guenette, S., Heymans, J. J., Walters, C. J., Watson, R., Zeller, D., et al. (2003). Hundred-year decline of North Atlantic predatory fishes. Fish Fish. 4, 1–24. doi: 10.1046/j.1467-2979.2003.00103.x
Collins, M., Sutherland, M., Bouwer, L., Cheong, S. M., and Tibig, L. (2019). IPCC SROCC—Extremes, Abrupt Changes and Managing Risks. IPCC Special Report on Ocean and Cryosphere in a Changing Climate, 2019-09-24. Geneva: IPCC.
Costello, M. J., Coll, M., Danovaro, R., Halpin, P., Ojaveer, H., and Miloslavich, P. (2010). A census of marine biodiversity knowledge, resources, and future challenges. PLoS One 5:e12110. doi: 10.1371/journal.pone.0012110
Cury, P., Shannon, L., and Shin, Y.-J. (2003). “The functioning of marine ecosystems: a fisheries perspective,” in Responsible Fisheries in the Marine Ecosystem, eds M. Sinclair and G. Valdimarson (Wallingford: CABIP), 103–123. doi: 10.1079/9780851996332.0103
Deng, J., and Jin, X. (2001). Dynamic characteristics of abundance and community structure of fishery species in the overwintering ground of the Bohai Sea. J. Nat. Res. 1, 42–46. doi: 10.3321/j.issn:1000-3037.2001.01.008
Ding, Q., Chen, X., Yu, W., and Chen, Y. (2017). An assessment of “fishing down marine food webs” in coastal states during 1955–2010. Acta Oceanol. Sin. 36, 43–50. doi: 10.1007/s13131-017-1003-5
Ding, Q., Chen, X., Yu, W., Tian, S., and Chen, Y. (2016). An evaluation of underlying mechanisms for “fishing down marine food webs”. Acta Oceanol. Sin. 35, 32–38. doi: 10.1007/s13131-016-0896-8
Dou, S. (2014). “Eels in china: species, fisheries, stock management and culture,” in Eels and Humans, eds K. Tsukamoto and M. Kuroki (Tokyo: Springer), 117–128. doi: 10.1007/978-4-431-54529-3_8
Ellis, N., Smith, S. J., and Pitcher, C. R. (2012). Gradient forests: calculating importance gradients on physical predictors. Ecology 93, 156–168. doi: 10.1890/11-0252.1
FAO-FIGIS (2019). Fisheries Global Information System (FIGIS). FI Institutional Websites. In: FAO Fisheries and Aquaculture Department. Available online at: http://www.fao.org/fishery/statistics/global-capture-production/query/en (accessed October 13, 2018).
Frederiksen, M., Edwards, M., Richardson, A. J., Halliday, N. C., and Wanless, S. (2006). From plankton to top predators: bottom-up control of a marine food web across four trophic levels. J. Anim. Ecol. 75, 1259–1268. doi: 10.1111/j.1365-2656.2006.01148.x
Free, C. M., Thorson, J. T., Pinsky, M. L., Oken, K. L., Wiedenmann, J., and Jensen, O. P. (2019). Impacts of historical warming on marine fisheries production. Science 363, 979–983. doi: 10.1126/science.aau1758
Goedegebuure, M., Melbourne-Thomas, J., Corney, S. P., Hindell, M. A., and Constable, A. J. (2017). Beyond big fish: the case for more detailed representations of top predators in marine ecosystem models. Ecol. Modell. 359, 182–192. doi: 10.1016/j.ecolmodel.2017.04.004
Graham, N. A. J., Jennings, S., MacNeil, M. A., Mouillot, D., and Wilson, S. K. (2015). Predicting climate-driven regime shifts versus rebound potential in coral reefs. Nature 518, 94–97. doi: 10.1038/nature14140
Halpern, B. S., Walbridge, S., Selkoe, K. A., Kappel, C. V., Micheli, F., D’Agrosa, C., et al. (2008). A global map of human impact on marine ecosystems. Science 319, 948–952. doi: 10.1126/science.1149345
Hare, S. R., and Mantua, N. J. (2000). Empirical evidence for North Pacific regime shifts in 1977 and 1989. Prog. Oceanogr. 47, 103–145. doi: 10.1016/S0079-6611(00)00033-1
Hobday, A. J., Arrizabalaga, H., Evans, K., Nicol, S., Young, J. W., and Weng, K. C. (2015). Impacts of climate change on marine top predators: advances and future challenges. Deep Sea Res. PT. II 113, 1–8. doi: 10.1016/j.dsr2.2015.01.013
Hsieh, C., Reiss, C. S., Hunter, J. R., Beddington, J. R., May, R. M., and Sugihara, G. (2006). Fishing elevates variability in the abundance of exploited species. Nature 443, 859–862. doi: 10.1038/nature05232
Jackson, J. B., Kirby, M. X., Berger, W. H., Bjorndal, K. A., Botsford, L. W., Bourque, B. J., et al. (2001). Historical overfishing and the recent collapse of coastal ecosystems. Science 293, 629–637. doi: 10.1126/science.1059199
Jin, X., Zhang, B., and Xue, Y. (2010). The response of the diets of four carnivorous fishes to variations in the Yellow Sea ecosystem. Deep Sea Res. PT. II 57, 996–1000. doi: 10.1016/j.dsr2.2010.02.001
Jung, K. H., Rahman, S. M., Kang, C., Park, S., Heon Lee, S., Je Park, H., et al. (2017). The influence of climate regime shifts on the marine environment and ecosystems in the East Asian Marginal Seas and their mechanisms. Deep Sea Res. PT. II 143, 110–120. doi: 10.1016/j.dsr2.2017.06.010
Kang, Y. S., Jung, S., Zuenko, Y., Choi, I., and Dolganova, N. (2012). Regional differences in the response of mesozooplankton to oceanographic regime shifts in the northeast Asian marginal seas. Prog. Oceanogr. 9, 120–134. doi: 10.1016/j.pocean.2011.11.012
Kim, Y. S., Jang, C. J., and Yeh, S. (2018). Recent surface cooling in the Yellow and East China Seas and the associated North Pacific climate regime shift. Cont. Shelf Res. 156, 43–54. doi: 10.1016/j.csr.2018.01.009
Lehodey, P., Alheit, J., Barange, M., Baumgartner, T., Beaugrand, G., Drinkwater, K., et al. (2006). Climate variability, fish and fisheries. J. Clim. 19, 5009–5030. doi: 10.1175/JCLI3898.1
Li, Z., Jin, X., Zhang, B., Zhou, Z., Shan, X., and Dai, F. (2012). Interannual variations in the population characteristics of the Pacific cod Gadus microcephalus in the Yellow Sea. Oceanol. Limn. Sin. 43, 924–931. doi: 10.11693/hyhz201205008008
Liang, C., Xian, W., and Pauly, D. (2020). Assessments of 15 exploited fish stocks in chinese, south korean and japanese waters using the CMSY and BSM Methods. Front. Mar. Sci. 7:623. doi: 10.3389/fmars.2020.00623
Liu, Y., Cheng, J., and Chen, Y. (2009). A spatial analysis of trophic composition: a case study of hairtail (Trichiurus japonicus) in the East China Sea. Hydrobiologia 632, 79–90. doi: 10.1007/s10750-009-9829-2
Ma, S., Cheng, J., Li, J., Liu, Y., Wan, R., and Tian, Y. (2019). Interannual to decadal variability in the catches of small pelagic fishes from China Seas and its responses to climatic regime shifts. Deep-Sea Res. PT. II. 159, 112–129. doi: 10.1016/j.dsr2.2018.10.005
Mu, Y., Yu, H., Chen, J., and Zhu, Y. (2007). A qualitative appraisal of China’ s efforts in fishing capacity management. J. Ocean. Univ. China. 6, 1–11. doi: 10.1007/s11802-007-0001-1
Myers, R. A., and Worm, B. (2003). Rapid worldwide depletion of predatory fish communities. Nature 423, 280–283. doi: 10.1038/nature01610
Myers, R. A., and Worm, B. (2005). Extinction, survival or recovery of large predatory fishes. Philos. Trans. R. Soc. Lond B Biol. Sci. 360, 13–20. doi: 10.1098/rstb.2004.1573
Ottersen, G., and Hjermann, D. Ø, and Stenseth, N. C. (2006). Changes in spawning stock structure strengthen the link between climate and recruitment in a heavily fished cod (Gadus morhua) stock. Fish. Oceanogr. 15, 230–243. doi: 10.1111/j.1365-2419.2006.00404.x
Pang, Y., Tian, Y., Fu, C., Wang, B., Li, J., Ren, Y., et al. (2018). Variability of coastal cephalopods in overexploited China Seas under climate change with implications on fisheries management. Fish. Res. 208, 22–33. doi: 10.1016/j.fishres.2018.07.004
Park, S., Chu, P. C., and Lee, J. (2011). Interannual-to-interdecadal variability of the Yellow Sea Cold Water Mass in 1967–2008: Characteristics and seasonal forcings. J. Mar. Syst. 87, 177–193. doi: 10.1016/j.jmarsys.2011.03.012
Pauly, D., Christensen, V. V., Dalsgaard, J., Froese, R., and Torres, F. J. (1998). Fishing down marine food webs. Science 279, 860–863. doi: 10.1126/science.279.5352.860
Pauly, D., and Zeller, D. (2016). Catch reconstructions reveal that global marine fisheries catches are higher than reported and declining. Nat. Commun. 7:10244. doi: 10.1038/ncomms10244
Pekcan-Hekim, Z., Urho, L., Auvinen, H., Heikinheimo, O., Lappalainen, J., Raitaniemi, J., et al. (2011). Climate warming and Pikeperch Year-Class catches in the Baltic Sea. Ambio 40, 447–456. doi: 10.1007/s13280-011-0143-7
Perry, R. I., Cury, P., Brander, K., Jennings, S., Möllmann, C., and Planque, B. (2010). Sensitivity of marine systems to climate and fishing: concepts, issues and management responses. J. Marine Syst. 79, 427–435. doi: 10.1016/j.jmarsys.2008.12.017
Planque, B., Fromentin, J., Cury, P., Drinkwater, K. F., Jennings, S., Perry, R. I., et al. (2010). How does fishing alter marine populations and ecosystems sensitivity to climate? J. Mar. Syst. 79, 403–417. doi: 10.1016/j.jmarsys.2008.12.018
R Core Team. (2018). R: a Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Revelle, W. (2017). Package ‘psych’- Procedures for Psychological, Psychometric, and Personality Research. Available online at: https://cran.r-project.org/web/packages/psych/index.html (accessed March 21, 2018).
Rochet, M., Trenkel, V. M., Carpentier, A., Coppin, F., De Sola, L. G., Léauté, J., et al. (2010). Do changes in environmental and fishing pressures impact marine communities? An. Empir. Assess. J. Appl. Ecol. 47, 741–750. doi: 10.1111/j.1365-2664.2010.01841.x
Rodionov, S. N. (2004). A sequential algorithm for testing climate regime shifts. Geophys. Res. Lett. 31:L09204. doi: 10.1029/2004GL019448
Rodionov, S. N. (2006). Use of prewhitening in climate regime shift detection. Geophys. Res. Lett. 33:L12707. doi: 10.1029/2006GL025904
Sun, B. (2009). The Current Situation and Conservation of Scomberomorus Niphonius in Yellow Sea and Bohai Bay. Beijing: Chinese Academy of Agricultural Sciences.
Sun, D., and Lin, Z. (2004). Variations of major commercial fish stocks and strategies for fishery management in Beubu Gulf. J. Trop. Oceanogr. 2, 62–68. doi: 10.3724/sp.j.1118.2012.00062
Szuwalski, C. S., Burgess, M. G., Costello, C., and Gaines, S. D. (2017). High fishery catches through trophic cascades in China. PNAS 114, 717–721. doi: 10.1073/pnas.1612722114
Then, A. Y., Hoenig, J. M., Hall, N. G., and Hewitt, D. A. (2015). Evaluating the predictive performance of empirical estimators of natural mortality rate using information on over 200 fish species. ICES J. Mar. Sci. 72, 82–92. doi: 10.1093/icesjms/fsu136
Thompson, R. M., Brose, U., Dunne, J. A., Hall, R. O., Hladyz, S., Kitching, R. L., et al. (2012). Food webs: reconciling the structure and function of biodiversity. Trends Ecol. Evol. 27, 689–697. doi: 10.1016/j.tree.2012.08.005
Tian, Y. (2009). Interannual–interdecadal variations of spear squid Loligo bleekeri abundance in the southwestern Japan Sea during 1975–2006: impact of the trawl fishing and recommendations for management under the different climate regimes. Fish. Res. 100, 78–85. doi: 10.1016/j.fishres.2009.06.005
Tian, Y., Kidokoro, H., Watanabe, T., and Iguchi, N. (2008). The late 1980s regime shift in the ecosystem of Tsushima warm current in the Japan/East Sea: evidence from historical data and possible mechanisms. Prog. Oceanogr. 77, 127–145. doi: 10.1016/j.pocean.2008.03.007
Tian, Y., Uchikawa, K., Ueda, Y., and Cheng, J. (2014). Comparison of fluctuations in fish communities and trophic structures of ecosystems from three currents around Japan: synchronies and differences. ICES J. Mar. Sci. 71, 19–34. doi: 10.1093/icesjms/fst169
Tian, Y., Ueno, Y., Suda, M., and Akamine, T. (2004). Decadal variability in the abundance of Pacific saury and its response to climatic/oceanic regime shifts in the northwestern subtropical Pacific during the last half century. J. Mar. Syst. 52, 235–257. doi: 10.1016/j.jmarsys.2004.04.004
Travers, M., Watermeyer, K., Shannon, L. J., and Shin, Y. J. (2010). Changes in food web structure under scenarios of overfishing in the southern Benguela: Comparison of the Ecosim and OSMOSE modelling approaches. J. Mar. Syst. 79, 101–111. doi: 10.1016/j.jmarsys.2009.07.005
Tu, C., Chen, K., and Hsieh, C. (2018). Fishing and temperature effects on the size structure of exploited fish stocks. Sci. Rep. 8:7132. doi: 10.1038/s41598-018-25403-x
Wan, R., Wu, Y., Huang, L., Zhang, J., Gao, L., and Wang, N. (2010). Fatty acids and stable isotopes of a marine ecosystem: Study on the Japanese anchovy (Engraulis japonicus) food web in the Yellow Sea. Deep Sea Res. PT. II 57, 1047–1057. doi: 10.1016/j.dsr2.2010.02.006
Wetzel, C. R., and Punt, A. E. (2015). Evaluating the performance of data-moderate and catch only assessment methods for U.S. west coast groundfish. Fish. Res. 171, 170–187. doi: 10.1016/j.fishres.2015.06.005
Yan, L., Li, J., Ling, J., Lin, L., and Li, S. (2005). Analysis on recent status of the fishery resources in the East China Sea. J. Zhejiang Univ. (Natural Science) 24, 303–307.
Yan, Y., Wang, T., Hou, G., Lu, H., and Jin, X. (2010). Feeding habits and monthly and ontogenetic diet shifts of the greater lizardfish, Sauida tumbil in the Beibu Gulf of the South China Sea. J. Fish. Chin. 34, 1089–1098. doi: 10.3724/SP.J.1231.2010.06877
Yuan, X., Liu, Z., Cheng, J., and Tian, Y. (2017). Impact of climate change on nekton community structure and some commercial species in the Offshore Area of the northern East China Sea in winter. Acta Ecol. Sin. 8, 2796–2808. doi: 10.5846/stxb201512222549
Zhai, L., Liang, C., and Pauly, D. (2020). Assessments of 16 exploited fish stocks in chinese waters using the CMSY and BSM methods. Front. Mar. Sci. 7:483993. doi: 10.3389/fmars.2020.483993
Zhang, B., Tang, Q., and Jin, X. (2007a). Functional groups pf fish assemblages and their major species at high trophic level in the East China Sea. J. Fish. Sci. Chin. 14, 939–949. doi: 10.3321/j.issn:1005-8737.2007.06.009
Zhang, Q., Cheng, J., Xu, H., Shen, X., Yu, G., and Zheng, Y. (2007b). Fishery Resources and Sustainable Utilization in the East China Sea. Shanghai: Fudan University Press.
Zhang, Q., and Yang, S. (2005). Species, geography distribution and resource of chondrichthian fishes of China. J. Xiamen Univ. (Natural Science) 44, 207–211. doi: 10.3321/j.issn:0438-0479.2005.z1.048
Zhao, X., Cui, L., Li, S., and Liu, X. (2020). Chinese Fishery Statistics. Beijing: China Agriculture Press.
Zhou, S., Punt, A. E., Smith, A. D. M., Ye, Y., Haddon, M., Dichmont, C. M., et al. (2018). An optimized catch-only assessment method for data poor fisheries. ICES J. Mar. Sci. 75, 964–976. doi: 10.1093/icesjms/fsx226
Keywords: China Seas, climate change, life history trait, over-exploitation, piscivorous fish, regime shift
Citation: Liu D, Tian Y, Ma S, Li J, Sun P, Ye Z, Fu C, Lan K and Zhou S (2021) Long-Term Variability of Piscivorous Fish in China Seas Under Climate Change With Implication for Fisheries Management. Front. Mar. Sci. 8:581952. doi: 10.3389/fmars.2021.581952
Received: 10 July 2020; Accepted: 06 May 2021;
Published: 10 June 2021.
Edited by:
Simone Libralato, National Institute of Oceanography and Applied Geophysics, ItalyReviewed by:
Brian R. MacKenzie, Technical University of Denmark, DenmarkDaniel Pauly, Sea Around Us, Canada
Copyright © 2021 Liu, Tian, Ma, Li, Sun, Ye, Fu, Lan and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongjun Tian, yjtian@ouc.edu.cn; Zhenjiang Ye, yechen@ouc.edu.cn
 Dan Liu
Dan Liu Yongjun Tian
Yongjun Tian Shuyang Ma
Shuyang Ma Jianchao Li1
Jianchao Li1  Peng Sun
Peng Sun Zhenjiang Ye
Zhenjiang Ye Caihong Fu
Caihong Fu Kuowei Lan
Kuowei Lan Shijie Zhou
Shijie Zhou