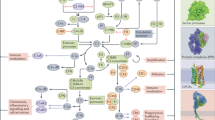
Abstract
Studies of complement genetics have changed the landscape of thrombotic microangiopathies (TMAs), particularly atypical haemolytic uraemic syndrome (aHUS). Knowledge of complement genetics paved the way for the design of the first specific treatment for aHUS, eculizumab, and is increasingly being used to aid decisions regarding discontinuation of anti-complement treatment in this setting. Complement genetic studies have also been used to investigate the pathogenic mechanisms that underlie other forms of HUS and provided evidence that contributed to the reclassification of pregnancy- and postpartum-associated HUS within the spectrum of complement-mediated aHUS. By contrast, complement genetics has not provided definite evidence of a link between constitutional complement dysregulation and secondary forms of HUS. Therefore, the available data do not support systematic testing of complement genes in patients with typical HUS or secondary HUS. The potential relevance of complement genetics for distinguishing the underlying mechanisms of malignant hypertension-associated TMA should be assessed with caution owing to the overlap between aHUS and other causes of malignant hypertension. In all cases, the interpretation of complement genetics results remains complex, as even complement-mediated aHUS is not a classical monogenic disease. Such interpretation requires the input of trained geneticists and experts who have a comprehensive view of complement biology.
Key points
-
Knowledge of complement genetics has transformed the landscape of atypical haemolytic uraemic syndrome (aHUS) and other forms of HUS.
-
To date, aHUS is the only form of HUS that has been clearly associated with genetic susceptibility factors related to complement regulation.
-
Pregnancy- and postpartum-associated HUS is part of the spectrum of complement-mediated HUS.
-
Secondary forms of HUS do not share genetic risk factors with aHUS.
-
Malignant hypertension is highly prevalent in patients with aHUS; however, aHUS is a rare cause of malignant hypertension.
-
Interpretation of complement genetics results requires comprehensive expertise in complement biology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Fakhouri, F., Zuber, J., Fremeaux-Bacchi, V. & Loirat, C. Haemolytic uraemic syndrome. Lancet 217, 681–696 (2017).
Warwicker, P. et al. Familial relapsing haemolytic uraemic syndrome and complement factor H deficiency. Nephrol. Dial. Transpl. 14, 1229–1233 (1999).
Richards, A. et al. Mutations in human complement regulator, membrane cofactor protein (CD46), predispose to development of familial hemolytic uremic syndrome. Proc. Natl Acad. Sci. USA 100, 12966–12971 (2003).
Noris, M. et al. Familial haemolytic uraemic syndrome and an MCP mutation. Lancet 362, 1542–1547 (2003).
Fremeaux-Bacchi, V. et al. The development of atypical haemolytic-uraemic syndrome is influenced by susceptibility factors in factor H and membrane cofactor protein: evidence from two independent cohorts. J. Med. Genet. 42, 852–856 (2005).
Fremeaux-Bacchi, V. et al. Complement factor I: a susceptibility gene for atypical haemolytic uraemic syndrome. J. Med. Genet. 41, e84 (2004).
Fremeaux-Bacchi, V. et al. Mutations in complement C3 predispose to development of atypical hemolytic uremic syndrome. Blood 112, 4948–4952 (2008).
Goicoechea de Jorge, E. et al. Gain-of-function mutations in complement factor B are associated with atypical hemolytic uremic syndrome. Proc. Natl Acad. Sci. USA 104, 240–245 (2007).
Fakhouri, F. et al. Terminal complement inhibitor eculizumab in adult patients with atypical hemolytic uremic syndrome: a single-arm, open-label trial. Am. J. Kidney Dis. 2016, 84–93 (2016).
Legendre, C. M. et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N. Engl. J. Med. 368, 2169–2181 (2013).
Bu, F. et al. Comprehensive genetic analysis of complement and coagulation genes in atypical hemolytic uremic syndrome. J. Am. Soc. Nephrol. 25, 55–64 (2014).
Fremeaux-Bacchi, V. et al. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin. J. Am. Soc. Nephrol. 8, 554–562 (2013).
Noris, M. et al. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin. J. Am. Soc. Nephrol. 5, 1844–1859 (2010).
Bu, F. et al. High-throughput genetic testing for thrombotic microangiopathies and C3 glomerulopathies. J. Am. Soc. Nephrol. 27, 1245–1253 (2016).
Osborne, A. J. et al. Statistical validation of rare complement variants provides insights into the molecular basis of atypical hemolytic uremic syndrome and C3 glomerulopathy. J. Immunol. 200, 2464–2478 (2018).
Merle, N. S., Church, S. E., Fremeaux-Bacchi, V. & Roumenina, L. T. Complement system Part I — molecular mechanisms of activation and regulation. Front. Immunol. 6, 262 (2015).
Fremeaux-Bacchi, V. et al. Complement gene variants and shiga toxin-producing escherichia coli-associated hemolytic uremic syndrome: retrospective genetic and clinical study. Clin. J. Am. Soc. Nephrol. 14, 364–377 (2019).
El Karoui, K. et al. Impact of hypertensive emergency and complement rare variants on presentation and outcome of atypical hemolytic uremic syndrome. Haematologica 104, 12 (2019).
Bu, F. et al. Genetic analysis of 400 patients refines understanding and implicates a new gene in atypical hemolytic uremic syndrome. J. Am. Soc. Nephrol. 29, 2809–2819 (2018).
Schaefer, F. et al. Clinical and genetic predictors of atypical hemolytic uremic syndrome phenotype and outcome. Kidney Int. 94, 408–418 (2018).
Le Quintrec, M. et al. Complement mutation-associated de novo thrombotic microangiopathy following kidney transplantation. Am. J. Transpl. 8, 1694–1701 (2008).
Le Clech, A. et al. Atypical and secondary hemolytic uremic syndromes have a distinct presentation and no common genetic risk factors. Kidney Int. 95, 1443–1452 (2019).
Jodele, S. et al. The genetic fingerprint of susceptibility for transplant-associated thrombotic microangiopathy. Blood 127, 989–996 (2016).
Ardissino, G. et al. Acquired complement regulatory gene mutations and hematopoietic stem cell transplant-related thrombotic microangiopathy. Biol. Blood Marrow Transpl. 23, 1580–1582 (2017).
Gavriilaki, E. et al. Pretransplant genetic susceptibility: clinical relevance in transplant-associated thrombotic microangiopathy. Thromb. Haemost. 120, 638–646 (2020).
Auton, A. et al. A global reference for human genetic variation. Nature. 526, 68–74 (2015).
Bruel, A. et al. Hemolytic uremic syndrome in pregnancy and postpartum. Clin. J. Am. Soc. Nephrol. 12, 1237–1247 (2017).
Gaggl, M. et al. Maternal and fetal outcomes of pregnancies in women with atypical hemolytic uremic syndrome. J. Am. Soc. Nephrol. 29, 1020–1029 (2018).
Huerta, A. et al. A retrospective study of pregnancy-associated atypical hemolytic uremic syndrome. Kidney Int. 93, 450–459 (2018).
Cavero, T. et al. Severe and malignant hypertension are common in primary atypical hemolytic uremic syndrome. Kidney Int. 96, 995–1004 (2019).
Vaught, A. J. et al. Germline mutations in the alternative pathway of complement predispose to HELLP syndrome. JCI Insight 3, e99128 (2018).
Fakhouri, F. et al. Factor H, membrane cofactor protein, and factor I mutations in patients with hemolysis, elevated liver enzymes, and low platelet count syndrome. Blood 112, 4542–4545 (2008).
Crovetto, F. et al. The genetics of the alternative pathway of complement in the pathogenesis of HELLP syndrome. J. Matern. Fetal Neonatal Med. 25, 2322–2325 (2012).
Arjona, E., Huerta, A., Goicoechea de Jorge, E. & Rodriguez de Cordoba, S. Familial risk of developing atypical hemolytic-uremic syndrome. Blood. 136, 1558–1561 (2020).
Lemaire, M. et al. Recessive mutations in DGKE cause atypical hemolytic-uremic syndrome. Nat. Genet. 45, 531–536 (2013).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17, 405–424 (2015).
Merinero, H. M., Garcia, S. P., Garcia-Fernandez, J., Arjona, E., Tortajada, A. & Rodriguez de Cordoba, S. Complete functional characterization of disease-associated genetic variants in the complement factor H gene. Kidney Int. 93, 470–481 (2017).
Marinozzi, M. C. et al. Complement factor B mutations in atypical hemolytic uremic syndrome-disease-relevant or benign? J. Am. Soc. Nephrol. 25, 2053–2065 (2014).
Seddon, J. M. et al. Rare variants in CFI, C3 and C9 are associated with high risk of advanced age-related macular degeneration. Nat. Genet. 45, 1366–1370 (2013).
Genomes Project, C. et al. A global reference for human genetic variation. Nature 526, 68–74 (2015).
Roumenina, L. T. et al. A prevalent C3 mutation in aHUS patients causes a direct C3 convertase gain of function. Blood 119, 4182–4191 (2012).
Gale, D. P. et al. Identification of a mutation in complement factor H-related protein 5 in patients of Cypriot origin with glomerulonephritis. Lancet 376, 794–801 (2010).
Noris, M. et al. Dynamics of complement activation in aHUS and how to monitor eculizumab therapy. Blood 124, 1715–1726 (2014).
Palomo, M. et al. Complement activation and thrombotic microangiopathies. Clin. J. Am. Soc. Nephrol. 14, 1719–1732 (2019).
Timmermans, S. et al. C5b9 formation on endothelial cells reflects complement defects among patients with renal thrombotic microangiopathy and severe hypertension. J. Am. Soc. Nephrol. 29, 2234–2243 (2018).
Galbusera, M. et al. An Ex vivo test of complement activation on endothelium for individualized eculizumab therapy in hemolytic uremic syndrome. Am. J. Kidney Dis. 74, 56–72 (2019).
Zuber, J. et al. Targeted strategies in the prevention and management of atypical HUS recurrence after kidney transplantation. Transpl. Rev. 27, 117–125 (2013).
Ardissino, G. et al. Discontinuation of eculizumab treatment in atypical hemolytic uremic syndrome: an update. Am. J. Kidney Dis. 66, 172–173 (2015).
Fakhouri, F. et al. Pathogenic variants in complement genes and risk of atypical hemolytic uremic syndrome relapse after eculizumab discontinuation. Clin. J. Am. Soc. Nephrol. 12, 50–59 (2017).
Wetzels, J. F. & van de Kar, N. C. Discontinuation of eculizumab maintenance treatment for atypical hemolytic uremic syndrome. Am. J. Kidney Dis. 65, 342 (2015).
Zuber, J. et al. Use of highly individualized complement blockade has revolutionized clinical outcomes after kidney transplantation and renal epidemiology of atypical hemolytic uremic syndrome. J. Am. Soc. Nephrol. 30, 2449–2463 (2019).
Fakhouri, F. et al. Eculizumab discontinuation in children and adults with atypical haemolytic uremic syndrome: a prospective multicentric study. Blood https://doi.org/10.1182/blood.2020009280 (2020).
Delvaeye, M. et al. Thrombomodulin mutations in atypical hemolytic-uremic syndrome. N. Engl. J. Med. 361, 345–357 (2009).
Holmes, L. V. et al. Determining the population frequency of the CFHR3/CFHR1 deletion at 1q32. PLoS One 8, e60352 (2013).
Dragon-Durey, M. A. et al. Clinical features of anti-factor H autoantibody-associated hemolytic uremic syndrome. J. Am. Soc. Nephrol. 21, 2180–2187 (2010).
Puraswani, M. et al. Clinical and immunological profile of anti-factor h antibody associated atypical hemolytic uremic syndrome: a nationwide database. Front. Immunol. 10, 1282 (2019).
Challis, R. C. et al. Thrombotic microangiopathy in inverted formin 2-mediated renal disease. J. Am. Soc. Nephrol. 28, 1084–1091 (2017).
Stahl, A. L. et al. A novel mutation in the complement regulator clusterin in recurrent hemolytic uremic syndrome. Mol. Immunol. 46, 2236–2243 (2009).
Azukaitis, K. et al. The phenotypic spectrum of nephropathies associated with mutations in diacylglycerol kinase epsilon. J. Am. Soc. Nephrol. 28, 3066–3075 (2017).
Bruneau, S. et al. Loss of DGKepsilon induces endothelial cell activation and death independently of complement activation. Blood 125, 1038–1046 (2015).
Grange, S. et al. Adult-onset renal thrombotic microangiopathy and pulmonary arterial hypertension in cobalamin C deficiency. Lancet 386, 1011–1012 (2015).
Canigral, C. et al. Eculizumab for the treatment of pregnancy-related atypical hemolytic uremic syndrome. Ann. Hematol. 93, 1421–1422 (2013).
Chua, J., Paizis, K., He, S. Z. & Mount, P. Suspected atypical haemolytic uraemic syndrome in two post-partum patients with foetal-death in utero responding to eculizumab. Nephrology 22, 18–22 (2017).
De Sousa Amorim, E., Blasco, M., Quintana, L., Sole, M., de Cordoba, S. R. & Campistol, J. M. Eculizumab in pregnancy-associated atypical hemolytic uremic syndrome: insights for optimizing management. J. Nephrol. 28, 641–645 (2015).
Delmas, Y., Bordes, C., Loirat, C., Fremeaux-Bacchi, V. & Combe, C. Post-partum atypical haemolytic-uraemic syndrome treated with eculizumab: terminal complement activity assessment in clinical practice. Clin. Kidney J. 6, 243–244 (2013).
Kourouklaris, A. et al. Postpartum thrombotic microangiopathy revealed as atypical hemolytic uremic syndrome successfully treated with eculizumab: a case report. J. Med. Case Rep. 8, 307 (2014).
Zschiedrich, S., Prager, E. P. & Kuehn, E. W. Successful treatment of the postpartum atypical hemolytic uremic syndrome with eculizumab. Ann. Intern. Med. 159, 76 (2013).
Bayer, G. et al. Etiology and outcomes of thrombotic microangiopathies. Clin. J. Am. Soc. Nephrol. 14, 557–566 (2019).
Frimat, M. et al. Renal cortical necrosis in postpartum hemorrhage: a case series. Am J Kidney Dis. 68, 50–57 (2016).
Haeger, M. et al. Increased release of tumor necrosis factor-alpha and interleukin-6 in women with the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Acta Obstet. Gynecol. Scand. 75, 695–701 (1996).
Vaught, A. J. et al. Direct evidence of complement activation in HELLP syndrome: A link to atypical hemolytic uremic syndrome. Exp. Hematol. 44, 390–398 (2016).
Salmon, J. E. et al. Mutations in complement regulatory proteins predispose to preeclampsia: a genetic analysis of the PROMISSE cohort. PLoS Med. 8, e1001013 (2011).
Penning, M. et al. Classical complement pathway activation in the kidneys of women with preeclampsia. Hypertension 66, 117–125 (2015).
Yonekura Collier, A. R. et al. Placental sFLT1 is associated with complement activation and syncytiotrophoblast damage in preeclampsia. Hypertens. Pregnancy 38, 193–199 (2019).
Bu, F. et al. Soluble c5b-9 as a biomarker for complement activation in atypical hemolytic uremic syndrome. Am. J. Kidney Dis. 65, 968–969 (2015).
Servais, A. et al. Atypical haemolytic uraemic syndrome and pregnancy: outcome with ongoing eculizumab. Nephrol. Dial. Transpl. 31, 2122–2130 (2016).
Kelly, R. J. et al. Eculizumab in pregnant patients with paroxysmal nocturnal hemoglobinuria. N. Engl. J. Med. 373, 1032–1039 (2015).
Cavero, T. et al. Eculizumab in secondary atypical haemolytic uraemic syndrome. Nephrol. Dial. Transpl. 32, 466–474 (2017).
Fakhouri, F. et al. Insights from the use in clinical practice of eculizumab in adult patients with atypical hemolytic uremic syndrome affecting the native kidneys: an analysis of 19 cases. Am. J. Kidney Dis. 63, 40–48 (2014).
Timmermans, S. et al. Patients with hypertension-associated thrombotic microangiopathy may present with complement abnormalities. Kidney Int. 91, 1420–1425 (2017).
van den Born, B. H. et al. ESC Council on hypertension position document on the management of hypertensive emergencies. Eur. Heart J. Cardiovasc. Pharmacother. 5, 37–46 (2019).
Timmermans, S. et al. Diagnostic and risk factors for complement defects in hypertensive emergency and thrombotic microangiopathy. Hypertension 75, 422–430 (2020).
Goodship, T. H. et al. Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 91, 539–551 (2017).
Rubin, S. et al. Malignant hypertension: diagnosis, treatment and prognosis with experience from the Bordeaux cohort. J. Hypertens. 37, 316–324 (2019).
El Karoui, K. et al. A clinicopathologic study of thrombotic microangiopathy in IgA nephropathy. J. Am. Soc. Nephrol. 23, 137–148 (2012).
Lane, D. A., Lip, G. Y. & Beevers, D. G. Improving survival of malignant hypertension patients over 40 years. Am. J. Hypertens. 22, 1199–1204 (2009).
Lip, G. Y., Beevers, M. & Beevers, D. G. Complications and survival of 315 patients with malignant-phase hypertension. J. Hypertens. 13, 915–924 (1995).
Polgreen, L. A., Suneja, M., Tang, F., Carter, B. L. & Polgreen, P. M. Increasing trend in admissions for malignant hypertension and hypertensive encephalopathy in the United States. Hypertension 65, 1002–1007 (2015).
van den Born, B. J., Koopmans, R. P., Groeneveld, J. O. & van Montfrans, G. A. Ethnic disparities in the incidence, presentation and complications of malignant hypertension. J. Hypertens. 24, 2299–2304 (2006).
Shantsila, A., Shantsila, E., Beevers, D. G. & Lip, G. Y. H. Predictors of 5-year outcomes in malignant phase hypertension: the West Birmingham Malignant Hypertension Registry. J. Hypertens. 35, 2310–2314 (2017).
Larsen, C. P., Wilson, J. D., Best-Rocha, A., Beggs, M. L. & Hennigar, R. A. Genetic testing of complement and coagulation pathways in patients with severe hypertension and renal microangiopathy. Mod. Pathol. 31, 488–494 (2018).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of this article.
Corresponding author
Ethics declarations
Competing interests
F.F. has received consultancy and/or speaker honoraria from Roche, Alexion, Apellis, Achillion, Novartis and Alnylam. V.F.-B has received fees from Alexion Pharmaceuticals, Roche, Apellis, Novartis and Baxter for invited lectures and/or board membership and is the recipient of a research grant from Alexion Pharmaceuticals and Apellis.
Additional information
Peer review information
Nature Reviews Nephrology thanks the anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Genome Aggregation Database (gnomAD): https://gnomad.broadinstitute.org/
Supplementary information
Glossary
- Next-generation sequencing
-
A high-throughput methodology that enables rapid sequencing of the base pairs in DNA samples.
- Sanger sequencing
-
This ‘first-generation’ DNA sequencing method is considered to be the gold standard for validating DNA sequences, including those that have been obtained using next-generation sequencing.
- Multiplex ligation-dependent probe amplification
-
A multiplex assay to detect copy number variations of genomic DNA sequences.
- Non-allelic homologous recombination
-
A molecular mechanism of exchange between two long segments of DNA (~300 bp or longer) that have very high sequence homology.
- Combined annotation dependent depletion
-
(CADD). An in silico tool that is designed to predict the pathogenicity of variants. CADD scores are based on diverse genomic features derived from the surrounding sequence context, gene model annotations, evolutionary constraint, epigenetic measurements and functional predictions. In silico predictive scores should be used, at most, as supporting evidence of pathogenicity.
Rights and permissions
About this article
Cite this article
Fakhouri, F., Frémeaux-Bacchi, V. Thrombotic microangiopathy in aHUS and beyond: clinical clues from complement genetics. Nat Rev Nephrol 17, 543–553 (2021). https://doi.org/10.1038/s41581-021-00424-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-021-00424-4
This article is cited by
-
Anti-factor B antibodies in atypical hemolytic uremic syndrome
Pediatric Nephrology (2024)
-
Severe hypertension and (renal) thrombotic microangiopathy: solving the puzzle
Journal of Nephrology (2023)
-
Genetic, clinical, and pathological study of patients with severe hypertension-associated renal microangiopathy
Journal of Nephrology (2023)
-
Variants in complement genes are uncommon in patients with anti-factor H autoantibody-associated atypical hemolytic uremic syndrome
Pediatric Nephrology (2023)
-
Clinical features and outcomes of patients with diacylglycerol kinase epsilon nephropathy: a nationwide experience
Pediatric Nephrology (2023)