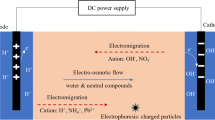
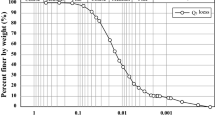
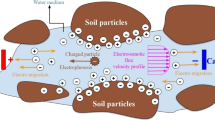
Abstract
Electrokinetic remediation technique is widely applied for the removal of heavy metal from contaminated soil, but the soil buffering capacity and fractionation of heavy metals mainly affect the cost and duration of the treatment. This study aims to treat heavy metal-contaminated sediments by electrokinetic remediation (EKR) technique by using various enhancing agents such as EDTA, \({HNO_3}\), HCI, \({H_2SO_4}\), acetic acid and citric acid for optimizing the cost and treatment duration. The optimum molar concentration of enhancing agent for treatment was estimated by batch experiments to maximize the dissolution of target heavy metals and reduce the dissolution of earth metals (Fe, Al and Ca) to maintain soil health. The EKR experiments were performed up to 15 days with the above enhancing agents to reduce the risk associated with heavy metals and the selection of enhancing agents based on removal efficiency was found to be in an order of EDTA > citric acid > acetic acid > \({HNO_3}\) > HCl \(\ge\) \({H_2SO_4}\). Also, a numerical model has been developed by incorporating main electrokinetic transport phenomena (electromigration and electroosmosis) and geochemical processes for the prediction of treatment duration and to scale up the EKR process. The model predicts well with experimental heavy metal removal with a MAPD of \(\approx\) 2-18 %. The parametric study on electrode distance for full-scale EKR treatment was found in this study as \(\approx\) 0.5 m.















Similar content being viewed by others
Data Availability Statement
Data will be made available on reasonable request.
References
Acar, Y. B., & Alshawabkeh, A. N. (1993). Principles of electrokinetic remediation. Environmental Science & Technology, 27(13), 2638–2647. https://doi.org/10.1021/es00049a002
Alshawabkeh, A. N., & Acar Y. B. (1992). Journal of Environmental Science and Health . Part A : Environmental Science and Engineering and Toxicology : Toxic / Hazardous Substances and Environmental Engineering Removal of contaminants from soils by electrokinetics : A theoretical treatise. Journal of environmental science and health, 27(7):1835–1861.
Alshawabkeh, A. N., Yeung, A. T., & Bricka, M. R. (1999). Practical Aspects of In-Situ Electrokinetic Extraction. Journal of Environmental Engineering, 125(1), 27–35. https://doi.org/10.1061/(ASCE)0733-9372(1999)125:1(27)
Alshawabkeh, A. N., Sheahan, T. C., & Wu, X. (2004). Coupling of electrochemical and mechanical processes in soils under DC fields. Mechanics of Materials, 36(5–6), 453–465. https://doi.org/10.1016/S0167-6636(03)00071-1
Amiard, J. C., Geffard, A., Amiard-Triquet, C., & Crouzet, C. (2007). Relationship between the lability of sediment-bound metals (Cd, Cu, Zn) and their bioaccumulation in benthic invertebrates. Estuarine, Coastal and Shelf Science, 72(3), 511–521. https://doi.org/10.1016/j.ecss.2006.11.017
Amrate, S., & Akretche, D. E. (2005). Modeling EDTA enhanced electrokinetic remediation of lead contaminated soils. Chemosphere, 60(10), 1376–1383. https://doi.org/10.1016/j.chemosphere.2005.02.021
Amrate, S., Akretche, D. E., Innocent, C., & Seta, P. (2005). Removal of Pb from a calcareous soil during EDTA-enhanced electrokinetic extraction. Science of The Total Environment, 349(1–3), 56–66. https://doi.org/10.1016/j.scitotenv.2005.01.018
Anderson, P. R., & Christensen, T. H. (1988). Distribution coefficients of Cd Co, Ni, and Zn in soils. Journal of Soil Science, 39(1), 15–22. https://doi.org/10.1111/j.1365-2389.1988.tb01190.x
Ayyanar, A., & Thatikonda, S. (2020a). Distribution and ecological risks of heavy metals in Lake Hussain Sagar, India. Acta Geochimica, 39(2), 255–270. https://doi.org/10.1007/s11631-019-00360-y
Ayyanar, A., & Thatikonda, S. (2020b). Enhanced electrokinetic remediation (EKR) for heavy metal-contaminated sediments focusing on treatment of generated effluents from EKR and recovery of EDTA. Water Environment Research p wer.1369. https://onlinelibrary.wiley.com/doi/abs/10.1002/wer.1369
Ayyanar, A., & Thatikonda, S. (2020c). Enhanced Electrokinetic Removal of Heavy Metals from a Contaminated Lake Sediment for Ecological Risk Reduction. Soil and Sediment Contamination: An International Journal, 00(00), 1–23. https://doi.org/10.1080/15320383.2020.1783510
Bahemmat, M., Farahbakhsh, M., & Kianirad, M. (2016). Humic substances-enhanced electroremediation of heavy metals contaminated soil. Journal of Hazardous Materials, 312:307–318. https://doi.org/10.1016/j.jhazmat.2016.03.038
Barlas, N., Akbulut, N., & AydoSan, M. (2005). Assessment of heavy metal residues in the sediment and water samples of Uluabat Lake, Turkey. Bulletin of Environmental Contamination and Toxicology, 74(2), 286–293. https://doi.org/10.1007/s00128-004-0582-y
Bassi, R., Prasher, S. O., & Simpson, B. K. (2000). Extraction of metals from a contaminated sandy soil using citric acid. Environmental Progress, 19(4), 275–282. https://doi.org/10.1002/ep.670190415
Belly, R. T., Lauff, J. J., & Goodhue, C. T. (1975). Degradation of Ethylenediaminetetraacetic Acid by Microbial Populations from an Aerated Lagoon. Applied Microbiology, 29(6), 787–794. https://doi.org/10.1128/AEM.29.6.787-794.1975
Bi, R., Schlaak, M., Siefert, E., Lord, R., & Connolly, H. (2011). Influence of electrical fields (AC and DC) on phytoremediation of metal polluted soils with rapeseed (Brassica napus) and tobacco (Nicotiana tabacum). Chemosphere, 83(3), 318–326. https://doi.org/10.1016/j.chemosphere.2010.12.052
Cameselle, C., & Gouveia, S. (2018). Phytoremediation of mixed contaminated soil enhanced with electric current. Journal of Hazardous Materials, 361:95–102. https://doi.org/10.1016/j.jhazmat.2018.08.062
Cameselle, C., & Pena, A. (2016). Enhanced electromigration and electro-osmosis for the remediation of an agricultural soil contaminated with multiple heavy metals. Process Safety and Environmental Protection, 104:209–217. https://doi.org/10.1016/j.psep.2016.09.002
Cameselle, C., & Reddy, K. R. (2012). Development and enhancement of electro-osmotic flow for the removal of contaminants from soils. Electrochimica Acta, 86:10–22. https://doi.org/10.1016/j.electacta.2012.06.121
Carolin, C. F., & Kumar, P. S., Saravanan, A., Joshiba, G. J., Naushad, M. (2017). Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. Journal of Environmental Chemical Engineering, 5(3), 2782–2799. https://doi.org/10.1016/j.jece.2017.05.029
Chauhan, G., Pant, K. K., & Nigam, K. D. P. (2015). Chelation technology: a promising green approach for resource management and waste minimization. Environ Sci: Processes Impacts, 17(1), 12–40. https://doi.org/10.1039/C4EM00559G
Covelo, E. F., Vega, F. A., & Andrade, M. L. (2007). Competitive sorption and desorption of heavy metals by individual soil components. Journal of Hazardous Materials, 140(1–2), 308–315. https://doi.org/10.1016/j.jhazmat.2006.09.018
Davydova, S. (2005) Heavy metals as toxicants in big cities. Microchemical Journal, 79(1–2), 133–136. https://doi.org/10.1016/j.microc.2004.06.010
Dermont, G., Bergeron, M., Mercier, G., & Richer-Laflche, M. (2008). Soil washing for metal removal: A review of physical/chemical technologies and field applications. Journal of Hazardous Materials, 152(1), 1–31. https://doi.org/10.1016/j.jhazmat.2007.10.043
Di Palma, L., & Mecozzi, R. (2007). Heavy metals mobilization from harbour sediments using EDTA and citric acid as chelating agents. Journal of Hazardous Materials, 147(3), 768–775. https://doi.org/10.1016/j.jhazmat.2007.01.072
Di Palma, L., Gonzini, O., & Mecozzi, R. (2011). Use of different chelating agents for heavy metal extraction from contaminated harbour sediment. Chemistry and Ecology, 27(sup1), 97–106. https://doi.org/10.1080/02757540.2010.534084
Diatta, J., Wirth, S., & Chudzińska, E. (2010). Application of the Partition Coefficient for Assessing Heavy Metals Mobility Within the Miasteczko Slaskie Zinc Smetler Impact Zone (Poland). Ecological Chemistry and Engineering A, 17(9), 1203–1212.
Egli, T. (2001). Biodegradation of Metal-Complexing Aminopolycarboxylic Acids. Journal of Bioscience and Bioengineering, 92(2), 89–97. https://doi.org/10.1263/jbb.92.89
EPA. (1999). Understanding variation in partition coefficient, Kd, values. Volume I: The Kd Model, Methods of Measurement, and Application of Chemical Reaction Codes.
Feng, H., Han, X., Zhang, W., & Yu, L. (2004). A preliminary study of heavy metal contamination in Yangtze River intertidal zone due to urbanization. Marine Pollution Bulletin, 49(11–12), 910–915. https://doi.org/10.1016/j.marpolbul.2004.06.014
Ganugapenta, S., Nadimikeri, J., Chinnapolla, S. R. R. B., Ballari, L., Madiga, R. K. N., & Tella, L. P. (2018). Assessment of heavy metal pollution from the sediment of Tupilipalem Coast, southeast coast of India. International Journal of Sediment Research, 33(3), 294–302. https://doi.org/10.1016/j.ijsrc.2018.02.004
Ghosh, M., & Singh, S. P. (2005). A comparative study of cadmium phytoextraction by accumulator and weed species. Environmental Pollution, 133(2), 365–371. https://doi.org/10.1016/j.envpol.2004.05.015
Giannis, A., Gidarakos, E., & Skouta, A. (2008). Transport of cadmium and assessment of phytotoxicity after electrokinetic remediation. Journal of Environmental Management, 86(3), 535–544. https://doi.org/10.1016/j.jenvman.2006.12.003
Giannis, A., Pentari, D., Wang, J. Y., & Gidarakos, E. (2010). Application of sequential extraction analysis to electrokinetic remediation of cadmium, nickel and zinc from contaminated soils. Journal of Hazardous Materials, 184(1–3), 547–554. https://doi.org/10.1016/j.jhazmat.2010.08.070
Gidarakos, E., & Giannis, A. (2006). Chelate agents enhanced electrokinetic remediation for removal cadmium and zinc by conditioning catholyte pH. Water, Air, and Soil Pollution, 172(1–4), 295–312. https://doi.org/10.1007/s11270-006-9080-7
Hanay, O., Hasar, H., & Kocer, N. N. (2009). Effect of EDTA as washing solution on removing of heavy metals from sewage sludge by electrokinetic. Journal of Hazardous Materials, 169(1–3), 703–710. https://doi.org/10.1016/j.jhazmat.2009.04.008
Henneken, L., Nörtemann, B., & Hempel, D. C. (1995). Influence of physiological conditions on EDTA degradation. Applied Microbiology and Biotechnology, 44(1–2), 190–197. https://doi.org/10.1007/s002530050540
Hinck, M. L., Ferguson, J., & Puhaakka, J. (1997). Resistance of EDTA and DTPA to aerobic biodegradation. Water Science and Technology, 35(2–3), 25–31. https://doi.org/10.1016/S0273-1223(96)00911-0
Ho, S. V., Athmer, C. J., Sheridan, P. W., & Shapiro, A. P. (1997). Scale-up aspects of the Lasagna® process for in situ soil decontamination. Journal of Hazardous Materials, 55(1–3), 39–60. https://doi.org/10.1016/S0304-3894(97)00016-2
Iannelli, R., Masi, M., Ceccarini, A., Ostuni, M. B., Lageman, R., Muntoni, A., Spiga, D., Polettini, A., Marini, A., & Pomi, R. (2015). Electrokinetic remediation of metal-polluted marine sediments: Experimental investigation for plant design. Electrochimica Acta, 181:146–159. https://doi.org/10.1016/j.electacta.2015.04.093
Ilyas, S., Anwar, M. A., Niazi, S. B., & Afzal, G. M. (2007) Bioleaching of metals from electronic scrap by moderately thermophilic acidophilic bacteria. Hydrometallurgy, 88(1–4), 180–188. https://doi.org/10.1016/j.hydromet.2007.04.007
Jain, C. K., Gurunadha, R. V. V. S., Prakash, B. A, Mahesh, K. K., Yoshida, M., & Kumar, B. A. P. K. M. (2010). Metal fractionation study on bed sediments of Hussainsagar Lake, Hyderabad, India. Environmental Monitoring and Assessment, 166(1–4), 57–67. https://doi.org/10.1007/s10661-009-0984-8
Kaushik, A., Kansal, A., Santosh, M., Kumari, S., & Kaushik, C. P. (2009). Heavy metal contamination of river Yamuna, Haryana, India: Assessment by Metal Enrichment Factor of the Sediments. Journal of Hazardous Materials, 164(1), 265–270. https://doi.org/10.1016/j.jhazmat.2008.08.031
Kim, C., Lee, Y., & Ong, S. K. (2003a). Factors affecting EDTA extraction of lead from lead-contaminated soils. Chemosphere, 51(9), 845–853. https://doi.org/10.1016/S0045-6535(03)00155-3
Kim, G. N., Yang, B. I., Moon, J. K., & Lee, K. W. (2009). Vertical electrokinetic-flushing remediation. Separation Science and Technology, 44(10), 2354–2370. https://doi.org/10.1080/01496390902983703
Kim, K. J., Kim, D. H., Yoo, J. C., & Baek, K. (2011). Electrokinetic extraction of heavy metals from dredged marine sediment. Separation and Purification Technology, 79(2), 164–169. https://doi.org/10.1016/j.seppur.2011.02.010
Kim, S., Kim, J., Kim, K., & Yun, S. (2005). Models and Experiments on Electrokinetic Removal of Pb(II) from Kaolinite Clay. Separation Science and Technology, 39(8), 1927–1951. https://doi.org/10.1081/SS-120030775
Kim, S. O., Kim, J. J., Yun, S. T., & Kim, K. W. (2003b). Numerical and experimental studies on Cadmium(II) transport in kaolinite clay under electrical fields. Water, Air and Soil Pollution, 150(Ii):135–162. https://doi.org/10.1023/A:1026181800685
Kinniburgh, D. G., van Riemsdijk, W. H., Koopal, L. K., Borkovec, M., Benedetti, M. F., & Avena, M. J. (1999). Ion binding to natural organic matter: competition, heterogeneity, stoichiometry and thermodynamic consistency. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 151(1–2), 147–166. https://doi.org/10.1016/S0927-7757(98)00637-2
Labanowski, J., Monna, F., Bermond, A., Cambier, P., Fernandez, C., Lamy, I., & van Oort, F. (2008). Kinetic extractions to assess mobilization of Zn, Pb, Cu, and Cd in a metal-contaminated soil: EDTA vs. citrate. Environmental Pollution, 152(3):693–701. https://linkinghub.elsevier.com/retrieve/pii/S0269749107003326
Li, C., Zhou, K., Qin, W., Tian, C., Qi, M., Yan, X., & Han, W. (2019). A Review on Heavy Metals Contamination in Soil: Effects, Sources, and Remediation Techniques. Soil and Sediment Contamination, 28(4), 380–394. https://doi.org/10.1080/15320383.2019.1592108
Li, S., & Ma, Y. (2014). Urbanization, Economic Development and Environmental Change. Sustainability, 6(8), 5143–5161. https://doi.org/10.3390/su6085143
Lim, T. T., Tay, J. H., Wang, & J. Y. (2004). Chelating-Agent-Enhanced Heavy Metal Extraction from a Contaminated Acidic Soil. Journal of Environmental Engineering, 130(1), 59–66. https://doi.org/10.1061/(ASCE)0733-9372(2004)130:1(59)
Lima, A. T., Hofmann, A., Reynolds, D., Ptacek, C. J., Van Cappellen, P., Ottosen, L. M., Pamukcu, S., Alshawabekh, A., O’Carroll, D. M., Riis, C., Cox, E., Gent, D. B., Landis, R., Wang, J., Chowdhury, A. I. A., Secord, E. L., Sanchez-Hachair, A., Carroll, D. M. O., Riis, C., Cox, E., Gent, D. B., Landis, R., Chowdhury, A. I. A., & Secord, E. L. (2017). Environmental Electrokinetics for a sustainable subsurface. Chemosphere, 181:122–133. https://doi.org/10.1016/j.chemosphere.2017.03.143
Liu, P., Jun, Z. H., Li, W. L, Hui, L. Z., Lin W. J., Qin, W. Y., Hua, J. L., Dong L., & Feng, Z. Y. (2011). Analysis of Heavy Metal Sources for Vegetable Soils from Shandong Province, China. Agricultural Sciences in China, 10(1), 109–119. https://doi.org/10.1016/S1671-2927(11)60313-1
Liu, S., Wang, Z., Zhang, Y., Liu, Y., Yuan, W., Zhang, T., Liu, Y., Li, P., He, L., & Chen, J. (2019). Distribution and partitioning of heavy metals in large anthropogenically impacted river, the Pearl River, China. Acta Geochimica, 38(2), 216–231. https://doi.org/10.1007/s11631-018-00309-7
Long, E. R., Macdonald, D. D., Smith, S. L., Calder, & F. D. (1995). Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environmental Management, 19(1), 81–97. https://doi.org/10.1007/BF02472006
Ma F, Peng C, Hou D, Wu B, Zhang Q, & Li F, Gu Q (2015). Citric acid facilitated thermal treatment: An innovative method for the remediation of mercury contaminated soil. Journal of Hazardous Materials, 300:546–552. https://doi.org/10.1016/j.jhazmat.2015.07.055
Mascia, M., Palmas, S., Polcaro, A. M., Vacca, A., & Muntoni, A. (2007). Experimental study and mathematical model on remediation of Cd spiked kaolinite by electrokinetics. Electrochimica Acta 52, (10 SPEC. ISS.):3360–3365. https://doi.org/10.1016/j.electacta.2006.04.066
Mascia, M., Vacca, A., & Palmas, S. (2015). Effect of surface equilibria on the electrokinetic behaviour of Pb and Cd ions in kaolinite. Journal of Chemical Technology and Biotechnology, 90(7), 1290–1298. https://doi.org/10.1002/jctb.4435
Masi, M. (2017). Electrokinetic remediation of heavy metal-contaminated marine sediments: experiments and modelling. January. https://doi.org/10.13131/unipi/etd/01122017-120456
Masi, M., Ceccarini, A., & Iannelli, R. (2017b). Multispecies reactive transport modelling of electrokinetic remediation of harbour sediments. Journal of Hazardous Materials, 326:187–196. https://doi.org/10.1016/j.jhazmat.2016.12.032
Meng, F., Xue, H., Wang, Y., Zheng, B., & Wang, J. (2018). Citric-acid preacidification enhanced electrokinetic remediation for removal of chromium from chromium-residue-contaminated soil. Environmental Technology (United Kingdom), 39(3), 356–362. https://doi.org/10.1080/09593330.2017.1301565
Moghadam, M. J., Moayedi, H., Sadeghi, M. M., & Hajiannia, A. (2016). A review of combinations of electrokinetic applications. Environmental Geochemistry and Health, 38(6), 1217–1227. https://doi.org/10.1007/s10653-016-9795-3
Mohamadi, S., Saeedi, M., & Mollahosseini, A. (2019). Enhanced electrokinetic remediation of mixed contaminants from a high buffering soil by focusing on mobility risk. Journal of Environmental Chemical Engineering, p 103470. https://doi.org/10.1016/j.jece.2019.103470
Müller, B. (1996). ChemEQL: a program to calculate chemical speciation equilibria titrations, dissolutions, precipitation, adsorption, simple kinetics, and pX-pY diagrams. Swiss Federal Institute for Environmental Science and Technology (EAWAG), Kastanienbaum, Switzerland.
Mulligan, C. N., Yong, R. N., Gibbs, & B. F. (2001). Surfactant-enhanced remediation of contaminated soil: A review. Engineering Geology, 60(1–4), 371–380. https://doi.org/10.1016/S0013-7952(00)00117-4
Narasimhan, B., & Sri Ranjan, R. (2000). Electrokinetic barrier to prevent subsurface contaminant migration: theoretical model development and validation. Journal of Contaminant Hydrology, 42(1), 1–17. https://doi.org/10.1016/S0169-7722(99)00089-3
Olivares-rieumont, S., De La Rosa, D., Lima, L., Graham, D. W., D’Alessandro, K., Borroto, J., Martínez, F., Sánchez, J., De, D., Lima, L., Graham, D. W., Alessandro, K. D., Borroto, J., Mart, F., & Sa, J. (2005). Assessment of heavy metal levels in Almendares River sediments - Havana City, Cuba. Water Research, 39(16), 3945–3953. https://doi.org/10.1016/j.watres.2005.07.011
Park, J. S., Kim, S. O., Kim, K. W., Kim, B. R., & Moon, S. H. (2003). Numerical analysis for electrokinetic soil processing enhanced by chemical conditioning of the electrode reservoirs. Journal of Hazardous Materials, 99(1), 71–88. https://doi.org/10.1016/S0304-3894(03)00038-4
Park, S. W., Lee, J. Y., Yang, J. S., Kim, K. J., & Baek, K. (2009). Electrokinetic remediation of contaminated soil with waste-lubricant oils and zinc. Journal of Hazardous Materials, 169(1–3), 1168–1172. https://doi.org/10.1016/j.jhazmat.2009.04.039
Pazos, M., Gouveia, S., Sanromán, M. A., & Cameselle, C. (2008). Electromigration of Mn, Fe, Cu and Zn with citric acid in contaminated clay. Journal of Environmental Science and Health, Part A, 43(8), 823–831. https://doi.org/10.1080/10934520801974004
Perin, G., Craboledda, L. D. F., Lucchese, M. G., Cirillo, R., & Dotta, L. (1985). Heavy Metal Speciation in the Sediments of Northern Adriatic Sea: A new Approach for Environmental Toxicity Determination.
Pociecha, M., Kastelec, D., & Lestan, D. (2011). Electrochemical EDTA recycling after soil washing of Pb, Zn and Cd contaminated soil. Journal of Hazardous Materials, 192(2), 714–721. https://doi.org/10.1016/j.jhazmat.2011.05.077
Qiao, J., Sun, H., Luo, X., Zhang, W., Mathews, S., & Yin, X. (2017). EDTA-assisted leaching of Pb and Cd from contaminated soil. Chemosphere, 167(1–3), 422–428. https://doi.org/10.1016/j.chemosphere.2016.10.034
Reddy, K. R., & Chinthamreddy, S. (2003). Sequentially Enhanced Electrokinetic Remediation of Heavy Metals in Low Buffering Clayey Soils. Journal of Geotechnical and Geoenvironmental Engineering, 129(3), 263–277. https://doi.org/10.1061/(ASCE)1090-0241(2003)129:3(263)
Rozas, F., & Castellote, M. (2012). Electrokinetic remediation of dredged sediments polluted with heavy metals with different enhancing electrolytes. Electrochimica Acta, 86, 102–109. https://doi.org/10.1016/j.electacta.2012.03.068
Ryu, B. G., Park, S. W., Baek, K., & Yang, J. S. (2009). Pulsed electrokinetic decontamination of agricultural lands around abandoned mines contaminated with heavy metals. Separation Science and Technology, 44(10), 2421–2436. https://doi.org/10.1080/01496390902983778
Schultz, D. S. (1997). Electroosmosis technology for soil remediation : Laboratory results, field trial, and economic modeling. Journal of Hazardous Materials, 55(1–3), 81–91. https://doi.org/10.1016/S0304-3894(97)00014-9
Yuan S. S., & Heng, F. Z. (2004). Bioleaching of marmatite flotation concentrate by Acidithiobacillus ferrooxidans. Hydrometallurgy, 75(1–4), 1–10. https://doi.org/10.1016/j.hydromet.2004.05.008
Smith, R. M., & Martell, A. E. (1976). Critical stability constants: inorganic complexes, vol 4. Springer.
Song, Y., Ammami, M. T., Benamar, A., Mezazigh, S., & Wang, H. (2016). Effect of EDTA, EDDS, NTA and citric acid on electrokinetic remediation of As, Cd, Cr, Cu, Ni, Pb and Zn contaminated dredged marine sediment. Environmental Science and Pollution Research, 23(11), 10577–10586. https://doi.org/10.1007/s11356-015-5966-5
Srinivasan, V., Seto, K. C., Emerson, R., & Gorelick, S. M. (2013). The impact of urbanization on water vulnerability: A coupled human-environment system approach for Chennai, India. Global Environmental Change, 23(1), 229–239. https://doi.org/10.1016/j.gloenvcha.2012.10.002
Sun, B., Zhao, F. J., Lombi, E., & McGrath, S. P. (2001). Leaching of heavy metals from contaminated soils using EDTA. Environmental Pollution, 113(2), 111–120. https://doi.org/10.1016/S0269-7491(00)00176-7
Suzuki, T., Niinae, M., Koga, T., Akita, T., Ohta, M., & Choso, T. (2014). EDDS-enhanced electrokinetic remediation of heavy metal-contaminated clay soils under neutral pH conditions. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 440, 145–150. https://doi.org/10.1016/j.colsurfa.2012.09.050
Swarnalatha, K., Letha, J., Ayoob, S., & Nair, A. G. (2015). Risk assessment of heavy metal contamination in sediments of a tropical lake. Environmental Monitoring and Assessment, 187(6), 1–14. https://doi.org/10.1007/s10661-015-4558-7
Tang, J., He, J., Xin, X., Hu, H., & Liu, T. (2018). Biosurfactants enhanced heavy metals removal from sludge in the electrokinetic treatment. Chemical Engineering Journal, 334(73), 2579–2592. https://doi.org/10.1016/j.cej.2017.12.010
Taylor, P., Alshawabkeh, A. N., Gale, R. J., Ozsu-acar, E., & Bricka, R. M. (1999). Optimization of 2-D Electrode Configuration for Electrokinetic Remediation. Soil and Sediment Contamination, 8(6), 617–635. https://doi.org/10.1080/10588339991339504
Thayalakumaran, T. A., Vogeler, I. A., Scotter, D. R. A., Percival, H. J. C., Robinson, B. H. A., & Clothier, B. E. (2003). Leaching of copper from contaminated soil following the application of EDTA. I. Repacked soil experiments and a model. Soil Research, 41(2), 323. http://www.publish.csiro.au/?paper=SR02059
Tiedje, J. M. (1975). Microbial degradation of ethylenediaminetetraacetate in soils and sediments. Journal of Applied Microbiology, 30(2), 327–329. https://doi.org/10.1128/AEM.30.2.327-329.1975
Vázquez, M. V., Vasco, D. A., Hernández-Luis, F., Grandoso, D., Lemus, M., Benjumea, D. M., & Arbelo, C. D. (2009). Electrokinetic study of the buffer capacity of some soils from Tenerife. Comparison with a volumetric technique. Geoderma, 148(3–4), 261–266. https://doi.org/10.1016/j.geoderma.2008.10.010
Villen-Guzman, M., Paz-Garcia, J. M., Rodriguez-Maroto, J. M., Gomez-Lahoz, C., & Garcia-Herruzo, F. (2014). Acid Enhanced Electrokinetic Remediation of a Contaminated Soil using Constant Current Density: Strong vs. Weak Acid. Separation Science and Technology, 49(10), 1461–1468. https://doi.org/10.1080/01496395.2014.898306
Villen-Guzman, M., Paz-Garcia, J. M., Amaya-Santos, G., Rodriguez-Maroto, J. M., Vereda-Alonso, C., & Gomez-Lahoz, C. (2015a). Effects of the buffering capacity of the soil on the mobilization of heavy metals. Equilibrium and kinetics. Chemosphere, 131, 78–84. https://doi.org/10.1016/j.chemosphere.2015.02.034
Villen-Guzman, M., Paz-Garcia, J. M., Rodriguez-Maroto, J. M., Garcia-Herruzo, F., Amaya-Santos, G., Gomez-Lahoz, C., & Vereda-Alonso, C. (2015b). Scaling-up the acid-enhanced electrokinetic remediation of a real contaminated soil. Electrochimica Acta, 181, 139–145. https://doi.org/10.1016/j.electacta.2015.02.067
Villen-Guzman, M., Gomez-Lahoz, C., Garcia-Herruzo, F., Vereda-Alonso, C., Paz-Garcia, J., & Rodriguez-Maroto, J. (2017). Specific Energy Requirements in Electrokinetic Remediation. Transport in Porous Media. https://doi.org/10.1007/s11242-017-0965-2
Virkutyte, J., Sillanpää, M., & Latostenmaa, P. (2002). Electrokinetic soil remediation - Critical overview. Science of the Total Environment, 289(1–3), 97–121. https://doi.org/10.1016/S0048-9697(01)01027-0
Vulava, V. M., & Seaman, J. C. (2000). Mobilization of Lead from Highly Weathered Porous Material by Extracting Agents. Environmental Science & Technology, 34(22), 4828–4834. https://doi.org/10.1021/es001295j
Wang, C., Hu, X., Chen, M. L., & Wu, Y. H. (2005). Total concentrations and fractions of Cd, Cr, Pb, Cu, Ni and Zn in sewage sludge from municipal and industrial wastewater treatment plants. Journal of Hazardous Materials, 119(1–3), 245–249. https://doi.org/10.1016/j.jhazmat.2004.11.023
Wang, J. Y., Huang, X. J., & Kao, J. C. M., Stabnikova, O. (2006). Removal of heavy metals from kaolin using an upward electrokinetic soil remedial (UESR) technology. Journal of Hazardous Materials, 136(3), 532–541. https://doi.org/10.1016/j.jhazmat.2006.01.029
Wang, L. F., Yang, L. Y., Kong, L. H., Li, S., Zhu, J. R., & Wang, Y. Q. (2014). Spatial distribution, source identification and pollution assessment of metal content in the surface sediments of Nansi Lake, China. Journal of Geochemical Exploration, 140:87–95. https://doi.org/10.1016/j.gexplo.2014.02.008
Wong, J. S., Hicks, R., & Probstein, R. F. (1997). EDTA-enhanced electroremediation of metal-contaminated soils. Journal of Hazardous Materials, 55(1–3), 61–79. https://doi.org/10.1016/S0304-3894(97)00008-3
Yang, J. S. S., Lee, J. Y., Baek, K., Kwon, T. S. S., Choi, J, & Young, J. (2009). Extraction behavior of As, Pb, and Zn from mine tailings with acid and base solutions. Journal of Hazardous Materials, 171(1–3), 443–451. https://doi.org/10.1016/j.jhazmat.2009.06.021
Yeung, A. T., & Gu, Y. Y. (2011). A review on techniques to enhance electrochemical remediation of contaminated soils. Journal of Hazardous Materials, 195, 11–29. https://doi.org/10.1016/j.jhazmat.2011.08.047
Yin, K., Giannis, A., Wong, A. S., & Wang, J. Y. (2014). EDTA-enhanced thermal washing of contaminated dredged marine sediments for heavy metal removal. Water, Air, and Soil Pollution, 225(8). https://doi.org/10.1007/s11270-014-2024-8
Yoo, J. C., Lee, C. D., Yang, J. S., & Baek, K. (2013). Extraction characteristics of heavy metals from marine sediments. Chemical Engineering Journal, 228(May), 688–699. https://doi.org/10.1016/j.cej.2013.05.029
Yoo, J. C., Yang, J. S., Jeon, E. K., & Baek, K. (2015). Enhanced-electrokinetic extraction of heavy metals from dredged harbor sediment. Environmental Science and Pollution Research, 22(13), 9912–9921. https://doi.org/10.1007/s11356-015-4155-x
Yoo, J. C, Lee, C., Lee, J. S., & Baek, K. (2017). Simultaneous application of chemical oxidation and extraction processes is effective at remediating soil Co-contaminated with petroleum and heavy metals. Journal of Environmental Management, 186, 314–319. https://doi.org/10.1016/j.jenvman.2016.03.016
Zhang, T., Zou, H., Ji, M., Li, X., Li, L., & Tang, T. (2014). Enhanced electrokinetic remediation of lead-contaminated soil by complexing agents and approaching anodes. Environmental Science and Pollution Research, 21(4), 3126–3133. https://doi.org/10.1007/s11356-013-2274-9
Zhang, Y., Chu, G., Dong, P., Xiao, J., Meng, Q., Baumgartel, M., Xu, B., & Hao, T. (2018). Enhanced electrokinetic remediation of lead- and cadmium-contaminated paddy soil by composite electrolyte of sodium chloride and citric acid. Journal of Soils and Sediments, 18(5), 1915–1924. https://doi.org/10.1007/s11368-017-1890-2
Zou, Z., Qiu, R., Zhang, W., Dong, H., Zhao, Z., Zhang, T., Wei, X., & Cai, X. (2009). The study of operating variables in soil washing with EDTA. Environmental Pollution, 157(1), 229–236. https://doi.org/10.1016/j.envpol.2008.07.009
Acknowledgements
The authors like to acknowledge the support from Frontier Areas of Science and Technology-centre of Excellence (FAST-CoE) in Sustainable Development at Indian Institute of Technology Hyderabad, funded by the Ministry of Human Resource Development, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Appendices
Appendix
List of Tables
Rights and permissions
About this article
Cite this article
Ayyanar, A., Thatikonda, S. Experimental and Numerical studies on remediation of mixed metal-contaminated sediments by electrokinetics focusing on fractionation changes. Environ Monit Assess 193, 316 (2021). https://doi.org/10.1007/s10661-021-09064-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-021-09064-4