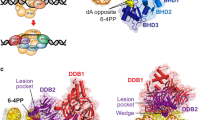
Abstract
In mammalian cells, base excision repair (BER) is the main pathway responsible for the correction of a variety of chemically modified DNA bases. DNA packaging in chromatin affects the accessibility of damaged sites to the enzymes involved in repair processes. This review presents data concerning the enzymes involved in BER. Within the nucleosome core particle (NCP), the accessibility of damaged DNA to enzymes is hindered by the presence of a histone octamer. This means that the removal of DNA lesions largely depends on their rotational and translational positioning in the NCP, as well as on the specific features of each enzyme.









Similar content being viewed by others
REFERENCES
Luger K., Mäder A.W., Richmond R.K., Sargent D.F., Richmond T.J. 1997. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature. 389, 251–260.
Vasudevan D., Chua E.Y.D., Davey C.A. 2010. Crystal structures of nucleosome core particles containing the “601” strong positioning sequence. J. Mol. Biol. 403, 1–10.
Finch J.T., Lutter L.C., Rhodes D., Brown R.S., Rushton B., Levitt M., Klug A. 1977. Structure of nucleosome core particles of chromatin. Nature. 269, 29–36.
Richmond T.J., Finch J.T., Rushton B., Rhodes D., Klug A. 1984. Structure of the nucleosome core particle at 7 Å resolution. Nature. 311, 532–537.
Davey C.A., Sargent D.F., Luger K., Maeder A.W., Richmond T.J. 2002. Solvent mediated interactions in the structure of the nucleosome core particle at 1.9 Å resolution. J. Mol. Biol. 319, 1097–1113.
Richmond T.J., Davey C.A. 2003. The structure of DNA in the nucleosome core. Nature. 423, 145–150.
Tsunaka Y., Kajimura N., Tate S., Morikawa K. 2005. Alteration of the nucleosomal DNA path in the crystal structure of a human nucleosome core particle. Nucleic Acids Res. 33, 3424–3434.
Tachiwana H., Kagawa W., Osakabe A., Kawaguchi K., Shiga T., Hayashi-Takanaka Y., Kimura H., Kurumizaka H. 2010. Structural basis of instability of the nucleosome containing a testis-specific histone variant, human H3T. Proc. Natl. Acad. Sci. U. S. A. 107, 10454–10459.
Ueda J., Harada A., Urahama T., Machida S., Maehara K., Hada M., Makino Y., Nogami J., Horikoshi N., Osakabe A., Taguchi H., Tanaka H., Tachiwana H., Yao T., Yamada M., et al. 2017. Testis-specific histone variant H3t gene is essential for entry into spermatogenesis. Cell Rep. 18, 593–600.
Harp J.M., Hanson B.L., Timm D.E., Bunick G.J. 2000. Asymmetries in the nucleosome core particle at 2.5 Å resolution. Acta Crystallogr. Sect. D Biol. Crystallogr. 56, 1513–1534.
White C.L., Suto R.K., Luger K. 2001. Structure of the yeast nucleosome core particle reveals fundamental changes in internucleosome interactions. EMBO J. 20, 5207–5218.
Clapier C.R., Chakravarthy S., Petosa C., Fernández-Tornero C., Luger K., Müller C.W. 2008. Structure of the Drosophila nucleosome core particle highlights evolutionary constraints on the H2A–H2B histone dimer. Proteins Struct. Funct. Genet. 71, 1–7.
Chua E.Y.D., Vasudevan D., Davey G.E., Wu B., Davey C.A. 2012. The mechanics behind DNA sequence-dependent properties of the nucleosome. Nucleic Acids Res. 40, 6338–6352.
Lowary P.T., Widom J. 1998. New DNA sequence rules for high affinity binding to histone octamer and sequence-directed nucleosome positioning. J. Mol. Biol. 276, 19–42.
Polach K.J., Widom J. 1999. Restriction enzymes as probes of nucleosome stability and dynamics. Methods Enzymol. 304, 278–298.
Simpson R.T., Stafford D.W. 1983. Structural features of a phased nucleosome core particle. Proc. Natl. Acad. Sci. U. S. A. 80, 51–55.
Long E.O., Dawid I.B. 1980. Repeated genes in eukaryotes. Annu. Rev. Biochem. 49, 727–764.
Pepenella S., Murphy K.J., Hayes J.J. 2014. Intra- and inter-nucleosome interactions of the core histone tail domains in higher-order chromatin structure. Chromosoma. 123, 3–13.
Mellor J. 2006. Dynamic nucleosomes and gene transcription. Trends Genet. 22 (6), 320–329. https://doi.org/10.1016/j.tig.2006.03.008
Studitsky V.M., Kassavetis G.A., Geiduschek E.P., Felsenfeldt G. 1997. Mechanism of transcription through the nucleosome by eukaryotic RNA polymerase. Science. 278, 1960–1963.
Kulaeva O.I., Gaykalova D.A., Pestov N.A., Golovastov V.V., Vassylyev D.G., Artsimovitch I., Studitsky V.M. 2009. Mechanism of chromatin remodeling and recovery during passage of RNA polymerase II. Nat. Struct. Mol. Biol. 16, 1272–1278.
Nacheva G.A., Guschin D.Y., Preobrazhenskaya O.V., Karpov V.L., Ebralidse K.K., Mirzabekov A.D. 1989. Change in the pattern of histone binding to DNA upon transcriptional activation. Cell. 58, 27–36.
Liu Y., Wilson S.H. 2012. DNA base excision repair: A mechanism of trinucleotide repeat expansion. Trends Biochem. Sci. 37, 162–172.
Esadze A., Stivers J.T. 2018. Facilitated diffusion mechanisms in DNA base excision repair and transcriptional activation. Chem. Rev. 118, 11298–11323.
Rodriguez Y., Hinz J.M., Smerdon M.J. 2015. Accessing DNA damage in chromatin: Preparing the chromatin landscape for base excision repair. DNA Repair (Amst.). 32, 113–119.
Jagannathan I., Cole H.A., Hayes J.J. 2006. Base excision repair in nucleosome substrates. Chromosom. Res. 14, 27–37.
Polach K.J., Widom J. 1995. Mechanism of protein access to specific DNA sequences in chromatin: a dynamic equilibrium model for gene regulation. J. Mol. Biol. 254, 130–149.
Li G., Levitus M., Bustamante C., Widom J. 2005. Rapid spontaneous accessibility of nucleosomal DNA. Nat. Struct. Mol. Biol. 12, 46–53.
Anderson J.D., Widom J. 2000. Sequence and position-dependence of the equilibrium accessibility of nucleosomal DNA target sites. J. Mol. Biol. 296, 979–987.
Tims H.S., Gurunathan K., Levitus M., Widom J. 2011. Dynamics of nucleosome invasion by DNA binding proteins. J. Mol. Biol. 411, 430–448.
Krokan H.E., Drabløs F., Slupphaug G. 2002. Uracil in DNA: Occurrence, consequences and repair. Oncogene. 21, 8935–8948.
Andersen S., Heine T., Sneve R., König I., Krokan H.E., Epe B., Nilsen H. 2004. Incorporation of dUMP into DNA is a major source of spontaneous DNA damage, while excision of uracil is not required for cytotoxicity of fluoropyrimidines in mouse embryonic fibroblasts. Carcinogenesis. 26, 547–555.
Kavli B., Otterlei M., Slupphaug G., Krokan H. 2007. Uracil in DNA: General mutagen, but normal intermediate in acquired immunity. DNA Repair (Amst.). 6, 505–516.
Lindahl T. 1993. Instability and decay of the primary structure of DNA. Nature. 362, 709–715.
Cortazar D., Kunz C., Saito Y., Steinacher R., Schar P. 2007. The enigmatic thymine DNA glycosylase. DNA Repair (Amst.). 6, 489–504.
Neddermann P., Jiricny J. 1993. The purification of a mismatch-specific thymine-DNA glycosylase from HeLa cells. J. Biol. Chem. 268, 21218–21224.
Neddermann P., Jiricny J. 1994. Efficient removal of uracil from G.U mispairs by the mismatch-specific thymine DNA glycosylase from HeLa cells. Proc. Natl. Acad. Sci. U. S. A. 91, 1642–1646.
Wibley J.E.A., Waters T.R., Haushalter K., Verdine G.L., Pearl L.H. 2003. Structure and specificity of the vertebrate anti-mutator uracil-DNA glycosylase SMUG1. Mol. Cell. 11, 1647–1659.
Masaoka A., Matsubara M., Hasegawa R., Tanaka T., Kurisu S., Terato H., Ohyama Y., Karino N., Matsuda A., Ide H. 2003. Mammalian 5-formyluracil−DNA glycosylase. 2. role of SMUG1 uracil−DNA glycosylase in repair of 5-formyluracil and other oxidized and deaminated base lesions. Biochemistry. 42, 5003–5012.
Hendrich B., Bird A. 1998. Identification and characterization of a family of mammalian methyl-CpG binding proteins. Mol. Cell. Biol. 18, 6538–6547.
Hendrich B., Hardeland U., Ng H.H., Jiricny J., Bird A. 1999. The thymine glycosylase MBD4 can bind to the product of deamination at methylated CpG sites. Nature. 401, 301–304.
Nilsen H., Lindahl T., Verreault A. 2002. DNA base excision repair of uracil residues in reconstituted nucleosome core particles. EMBO J. 21, 5943–5952.
Beard B.C., Wilson S.H., Smerdon M.J. 2003. Suppressed catalytic activity of base excision repair enzymes on rotationally positioned uracil in nucleosomes. Proc. Natl. Acad. Sci. U. S. A. 100, 7465–7470.
Beard B.C., Stevenson J.J., Wilson S.H., Smerdon M.J. 2005. Base excision repair in nucleosomes lacking histone tails. DNA Repair (Amst.). 4, 203–209.
Ishibashi T., So K., Cupples C.G., Ausio J. 2008. MBD4-mediated glycosylase activity on a chromatin template is enhanced by acetylation. Mol. Cell. Biol. 28, 4734–4744.
Cole H.A., Tabor-Godwin J.M., Hayes J.J. 2010. Uracil DNA glycosylase activity on nucleosomal DNA depends on rotational orientation of targets. J. Biol. Chem. 285, 2876–2885
Hinz J.M., Rodriguez Y., Smerdon M.J. 2010. Rotational dynamics of DNA on the nucleosome surface markedly impact accessibility to a DNA repair enzyme. Proc. Natl. Acad. Sci. U. S. A. 107, 4646–4651.
Kosmoski J.V., Smerdon M.J. 1999. Synthesis and nucleosome structure of DNA containing a UV photoproduct at a specific site. Biochemistry. 38, 9485–9494.
Rodriguez Y., Smerdon M.J. 2013. The structural location of DNA lesions in nucleosome core particles determines accessibility by base excision repair enzymes. J. Biol. Chem. 288, 13863–13875.
Tarantino M.E., Dow B.J., Drohat A.C., Delaney S. 2018. Nucleosomes and the three glycosylases: high, medium, and low levels of excision by the uracil DNA glycosylase superfamily. DNA Repair (Amst.). 72, 56–63.
Olsen L.C., Aasland R., Wittwer C.U., Krokan H.E., Helland D.E. 1989. Molecular cloning of human uracil-DNA glycosylase, a highly conserved DNA repair enzyme. EMBO J. 8, 3121–3125.
Xiao G., Tordova M., Jagadeesh J., Drohat A.C., Stivers J.T., Gilliland G.L. 1999. Crystal structure of Escherichia coli uracil DNA glycosylase and its complexes with uracil and glycerol: Structure and glycosylase mechanism revisited. Proteins Struct. Funct. Genet. 35, 13–24.
Wu B., Mohideen K., Vasudevan D., Davey C.A. 2010. Structural insight into the sequence dependence of nucleosome positioning. Structure. 18, 528–536.
Olmon E.D., Delaney S. 2017. Differential ability of five DNA glycosylases to recognize and repair damage on nucleosomal DNA. ACS Chem. Biol. 12, 692–701.
Hazra T.K., Izumi T., Boldogh I., Imhoff B., Kow Y.W., Jaruga P., Dizdaroglu M., Mitra S. 2002. Identification and characterization of a human DNA glycosylase for repair of modified bases in oxidatively damaged DNA. Proc. Natl. Acad. Sci. U. S. A. 99, 3523–3528.
Bandaru V., Sunkara S., Wallace S.S., Bond J.P. 2002. A novel human DNA glycosylase that removes oxidative DNA damage and is homologous to Escherichia coli endonuclease VIII. DNA Repair (Amst.). 1, 517–529.
Doublie S., Bandaru V., Bond J.P., Wallace S.S. 2004. The crystal structure of human endonuclease VIII-like 1 (NEIL1) reveals a zincless finger motif required for glycosylase activity. Proc. Natl. Acad. Sci. U. S. A. 101, 10284–10289.
Yang N., Chaudhry M.A., Wallace S.S. 2006. Base excision repair by hNTH1 and hOGG1: a two edged sword in the processing of DNA damage in γ-irradiated human cells. DNA Repair (Amst.). 5, 43–51.
Ide H., Kotera M. 2004. Human DNA glycosylases involved in the repair of oxidatively damaged DNA. Biol. Pharm. Bull. 27, 480–485.
Miyabe I., Zhang Q.-M., Kino K., Sugiyama H., Takao M., Yasui A., Yonei S. 2002. Identification of 5‑formyluracil DNA glycosylase activity of human hNTH1 protein. Nucleic Acids Res. 30, 3443–3448.
Prasad A., Wallace S.S., Pederson D.S. 2007. Initiation of base excision repair of oxidative lesions in nucleosomes by the human, bifunctional DNA glycosylase NTH1. Mol. Cell. Biol. 27, 8442–8453.
Liu X., Choudhury S., Roy R. 2003. In vitro and in vivo dimerization of human endonuclease III stimulates its activity. J. Biol. Chem. 278, 50061–50069.
Odell I.D., Newick K., Heintz N.H., Wallace S.S., Pederson D.S. 2010. Non-specific DNA binding interferes with the efficient excision of oxidative lesions from chromatin by the human DNA glycosylase, NEIL1. DNA Repair (Amst.). 9, 134–143.
Dou H., Mitra S., Hazra T.K. 2003. Repair of oxidized bases in DNA bubble structures by human dNA glycosylases NEIL1 and NEIL2. J. Biol. Chem. 278, 49679–49684.
Dou H., Theriot C.A., Das A., Hegde M.L., Matsumoto Y., Boldogh I., Hazra T.K., Bhakat K.K., Mitra S. 2008. Interaction of the human DNA glycosylase NEIL1 with proliferating cell nuclear antigen: the potential for replication-associated repair of oxidized bases in mammalian genomes. J. Biol. Chem. 283, 3130–3140.
Hegde M.L., Theriot C.A., Das A., Hegde P.M., Guo Z., Gary R.K., Hazra T.K., Shen B., Mitra S. 2008. Physical and functional interaction between human oxidized base-specific DNA glycosylase NEIL1 and flap endonuclease. J. Biol. Chem. 283, 27028–27037.
Odell I.D., Barbour J.-E., Murphy D.L., Della-Maria J.A., Sweasy J.B., Tomkinson A E., Wallace S.S., Pederson D.S. 2011. Nucleosome disruption by DNA ligase III-XRCC1 promotes efficient base excision repair. Mol. Cell. Biol. 31, 4623–4632.
Maher R.L., Prasad A., Rizvanova O., Wallace S.S., Pederson D.S. 2013. Contribution of DNA unwrapping from histone octamers to the repair of oxidatively damaged DNA in nucleosomes. DNA Repair (Amst.). 12, 964–971.
Maher R.L., Wallace S.S., Pederson D.S. 2019. The lyase activity of bifunctional DNA glycosylases and the 3'-diesterase activity of APE1 contribute to the repair of oxidized bases in nucleosomes. Nucleic Acids Res. 47, 2922–2931.
Fromme J.C., Bruner S.D., Yang W., Karplus M., Verdine G.L. 2003. Product-assisted catalysis in base-excision DNA repair. Nat. Struct. Mol. Biol. 10, 204–211.
Fromme J.C., Verdine G.L. 2003. DNA lesion recognition by the bacterial repair enzyme MutM. J. Biol. Chem. 278, 51543–51548.
Rydberg B., Lindahl T. 1982. Nonenzymatic methylation of DNA by the intracellular methyl group donor S‑adenosyl-L-methionine is a potentially mutagenic reaction. EMBO J. 1, 211–216.
Marnett L.J. 2002. Oxy radicals, lipid peroxidation and DNA damage. Toxicology. 181–182, 219–222.
Dosanjh M.K., Chenna A., Kim E., Fraenkel-Conrat H., Samson L., Singer B. 1994. All four known cyclic adducts formed in DNA by the vinyl chloride metabolite chloroacetaldehyde are released by a human DNA glycosylase. Proc. Natl. Acad. Sci. U. S. A. 91, 1024–1028.
Rydberg B., Qiu Z.H., Dosanjh M.K., Singer B. 1992. Partial purification of a human DNA glycosylase acting on the cyclic carcinogen adduct 1,N 6-ethenodeoxyadenosine. Cancer Res. 52, 1377–1179.
O’Brien P.J., Ellenberger T. 2004. Dissecting the broad substrate specificity of human 3-methyladenine-DNA glycosylase. J. Biol. Chem. 279, 9750–9757.
O’Connor T.R. 1993. Purification and characterization of human 3-methyladenine-DNA glycosylase. Nucleic Acids Res. 21, 5561–5569.
Kennedy E.E., Li C., Delaney S. 2019. Global repair profile of human alkyladenine DNA glycosylase on nucleosomes reveals DNA packaging effects. ACS Chem. Biol. 14, 1687–1692.
Wang Y. 2008. Bulky DNA lesions induced by reactive oxygen species. Chem. Res. Toxicol. 21, 276–281.
Zuo S., Boorstein R.J., Teebor G.W. 1995. Oxidative damage to 5-methylcytosine in DNA. Nucleic Acids Res. 23, 3239–3243.
Douki T., Delatour T., Paganon F., Cadet J. 1996. Measurement of oxidative damage at pyrimidine bases in γ-irradiated DNA. Chem. Res. Toxicol. 9, 1145–1151.
Bjelland S., Seeberg E. 2003. Mutagenicity, toxicity and repair of DNA base damage induced by oxidation. Mutat. Res. 531, 37–80.
Zhang Y., Yuan F., Wu X., Taylor J.S., Wang Z. 2001. Response of human DNA polymerase iota to DNA lesions. Nucleic Acids Res. 29, 928–935.
Hanes J.W., Thal D.M., Johnson K.A. 2006. Incorporation and replication of 8-oxo-deoxyguanosine by the human mitochondrial DNA polymerase. J. Biol. Chem. 281, 36241–36248.
Menoni H., Shukla M.S., Gerson V., Dimitrov S., Angelov D. 2012. Base excision repair of 8-oxoG in dinucleosomes. Nucleic Acids Res. 40, 692–700.
Cannan W.J., Tsang B.P., Wallace S.S., Pederson D.S. 2014. Nucleosomes suppress the formation of double-strand DNA breaks during attempted base excision repair of clustered oxidative damages. J. Biol. Chem. 289, 19881–19893.
Hinz J.M., Mao P., McNeill D.R., Wilson D.M. 2015. Reduced nuclease activity of apurinic/apyrimidinic endonuclease (APE1) variants on nucleosomes: Identification of access residues. J. Biol. Chem. 290, 21067–21075.
Cannan W.J., Rashid I., Tomkinson A.E., Wallace S.S., Pederson D.S. 2017. The human ligase IIIα-XRCC1 protein complex [erforms DNA nick repair after transient unwrapping of nucleosomal DNA. J. Biol. Chem. 292, 5227–5238.
Lee T.H. 2019. Physical chemistry of epigenetics: single-molecule investigations. J. Phys. Chem. B. 123, 8351–8362.
Li C., Delaney S. 2019. Histone H2A variants enhance the initiation of base excision repair in nucleosomes. ACS Chem. Biol. 14, 1041–1050.
Zlatanova J., Thakar A. 2008. H2A.Z: view from the top. Structure. 16, 166–179.
Billon P., Côté J. 2012. Precise deposition of histone H2A.Z in chromatin for genome expression and maintenance. Biochim. Biophys. Acta—Gene Regul. Mech. 1819, 290–302.
Yu Y., Deng Y., Reed S.H., Millar C.B., Waters R. 2013. Histone variant Htz1 promotes histone H3 acetylation to enhance nucleotide excision repair in Htz1 nucleosomes. Nucleic Acids Res. 41, 9006–9019.
Bowman G.D., Poirier M.G. 2015. Post-translational modifications of histones that influence nucleosome dynamics. Chem. Rev. 115, 2274–2295.
North J.A., Shimko J.C., Javaid S., Mooney A.M., Shoffner M.A., Rose S.D., Bundschuh R., Fishel R., Ottesen J.J., Poirier M.G. 2012. Regulation of the nucleosome unwrapping rate controls DNA accessibility. Nucleic Acids Res. 40, 10215–10227.
Michishita E., McCord R.A., Boxer L.D., Barber M.F., Hong T., Gozani O., Chua K.F. 2009. Cell cycle-dependent deacetylation of telomeric histone H3 lysine K56 by human SIRT6. Cell Cycle. 8, 2664–2666.
Maas N.L., Miller K.M., DeFazio L.G., Toczyski D.P. 2006. Cell cycle and checkpoint regulation of histone H3 K56 acetylation by Hst3 and Hst4. Mol. Cell. 23, 109–119.
Rodriguez Y., Horton J.K., Wilson S.H. 2019. Histone H3 lysine 56 acetylation enhances AP endonuclease 1-mediated repair of AP sites in nucleosome core particles. Biochemistry. 58, 3646–3655.
Prasad R., Liu Y., Deterding L.J., Poltoratsky V.P., Kedar P.S., Horton J.K., Kanno S.I., Asagoshi K., Hou E.W., Khodyreva S.N., Lavrik O.I., Tomer K.B., Yasui A., Wilson S.H. 2007. HMGB1 is a cofactor in mammalian base excision repair. Mol. Cell. 27, 829–841.
Bennett S.E., Sanderson R.J., Mosbaugh D.W. 1995. Processivity of Escherichia coli and rat liver mitochondrial uracil-DNA glycosylase is affected by NaCl concentration. Biochemistry. 34, 6109–6119.
Lau A.Y., Wyatt M.D., Glassner B.J., Samson L.D., Ellenberger T. 2000. Molecular basis for discriminating between normal and damaged bases by the human alkyladenine glycosylase AAG. Proc. Natl. Acad. Sci. U. S. A. 97, 13573–13578.
Asahara H., Wistort P.M., Bank J.F., Bakerian R.H., Cunningham R.P. 1989. Purification and characterization of Escherichia coli endonuclease III from the cloned nth gene. Biochemistry. https://doi.org/10.1021/bi00436a048
Widlund H.R., Vitolo J.M., Thiriet C., Hayes J.J. 2000. DNA sequence-dependent contributions of core histone tails to nucleosome stability: Differential effects of acetylation and proteolytic tail removal. Biochemistry. 39, 3835–3841.
Yang Z., Zheng C., Hayes J.J. 2007. The core histone tail domains contribute to sequence-dependent nucleosome positioning. J. Biol. Chem. 282, 7930–7938.
Funding
The work was supported by the Ministry of Science and Higher Education of the Russian Federation (project no. MD-3775.2019.4) and partially supported by budgetary funding (project no. АААА-А17-117020210022-4).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare they have no conflicts of interests.
The article contains no studies involving animals or humans as subjects of the study.
Additional information
Translated by N. Onishchenko
Rights and permissions
About this article
Cite this article
Kladova, O.A., Kuznetsov, N.A. & Fedorova, O.S. Initial stages of DNA Base Excision Repair in Nucleosomes. Mol Biol 55, 167–181 (2021). https://doi.org/10.1134/S0026893321020096
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893321020096