Abstract
Intensified vehicular traffic causes increased heavy metal contamination of the environment. We investigated the heavy metal chemistry of soils located under silver fir stands in the vicinity of Poland’s S7 roadway. Three sampling sites were located in fir stands in central Poland. Fieldwork included soil sampling of the organic (O) horizon and mineral (A) topsoil. We analyzed the soil pH, carbon (C) and nitrogen (N) concentration, and the HCl-extractable forms of sodium (Na) and heavy metals: copper (Cu), nickel (Ni), lead (Pb), and zinc (Zn). The stoichiometric ratios Cu:C, Ni:C, Pb:C, and Zn:C were also calculated. In all sites, a higher Na concentration was found in the 0–10 m from the forest edge. This zone was characterized by increased pH in the O horizon, increased Zn and Ni in the A horizon, and a decreased Pb in the O horizon. There was no clear pattern for the Cu concentration. The Ni:C and Zn:C ratios were correlated with pH, while Pb:C and Cu:C ratios were correlated with the clay minerals. HCl-extractable Ni and Zn concentrations in A horizon were greater near the roadway, revealing strong pH dependency. The roadway affects the geochemical background of the topsoil in the nearby fir stands. Mechanistically, we suggest that Na increases the soil pH and therefore enhances the ability of soil organic matter to bind Ni and Zn by releasing hydrogen from soil organic matter functional groups into the soil solution. A depleted Pb near the road was likely owing to the strong competition from Na.
Similar content being viewed by others
1 Introduction
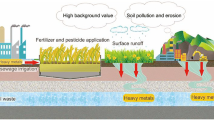
Progressive economic development led to an enlarged road network and intensified road transport. These increases have had a considerable impact on the natural environment, causing numerous ecological effects, e.g., loss of habitat, runoff emissions, and increased plant mortality (Spellberg, 1998). A severe consequence of the growing vehicular traffic is the increasing amount of heavy metal contamination in the environment. Heavy metals can enter the food chain, posing a real threat to humans, plants, and animals (Chrzan, 2013; Viard et al., 2004; Ward et al., 1974). Moreover, traffic is considered to be the main source of heavy metals in the atmosphere (Zereini et al., 2005). Therefore, it is crucial to thoroughly explore its ecological impact on all elements of the ecosystem.
The soil environment is an integral part of the ecosystem and is highly susceptible to heavy metal accumulation. The process of soil remediation is relatively slow compared with the remediation of air and water (Kowalik, 2001). The emerging interest in emissions related to vehicular traffic has been accompanied by the development of highways. Research in this field has been carried out for over 40 years (Laxen & Harrison, 1977; Motto et al., 1970; Warren & Birch, 1987) and has proven that pollutant concentrations decrease with increasing distance from roads, with increasing soil depth, and with decreasing traffic. The distance of the road’s impact zone on the soil has varied considerably between studies. Most related studies have found that the concentrations of heavy metals exceed the geochemical background within 5–10 m from the road edge (e.g., Bäckström et al., 2004; Grigalaviciene et al., 2005; Zehetner et al., 2009). Motor vehicles pose a growing threat of contaminating soils with heavy metals, which results in emerging contaminated zonal soil areas (Chrzan, 2013; Reimann & de Caritat, 2005). The most common heavy metals among traffic-originated pollutants are cadmium (Cd), chromium (Cr), copper (Cu), manganese (Mn), nickel (Ni), lead (Pb), and zinc (Zn) (Nriagu & Pacyna, 1988; Soleimani et al., 2018; Wong et al., 2006). Traffic-related emissions of heavy metals primarily originate from the deterioration of vehicles and their parts (e.g., wear to brake linings, the body, engine, clutch, and tires), as well as from oil leakage, inefficient fuel combustion, and exhaust systems (Councell et al., 2004; Kelly et al., 1996; Lindgren, 1996; Tossavainen & Forssberg, 1999; Wong et al., 2006). According to Zehetner et al. (2019), the accumulation of heavy metals originating from transport is much higher than the accumulation of metals from the geochemical background. Numerous studies in the traffic pollution literature have investigated the total forms of heavy metals, extractable by hydrogen fluoride, nitric acid, or aqua regia (Werkenthin et al., 2014). However, the pool of geochemically active heavy metals adsorbed onto the surface of the soil solid phase is only a fraction of the total forms and is the object of most geochemical studies focusing on contamination issues.
The primary factor that potentially modifies the geochemical background of soils in the vicinity of roadways is the inflow of heavy metals directly from traffic, but this background is also modified by the influx of sodium (Na) ions from de-icing agents. De-icing the roads in winter is a seasonal pollution source, mainly in the form of sodium chloride (NaCl) and magnesium chloride (CaCl2). Annually in temperate and cold climates, the winter road maintenance is a process responsible for emitting significant amounts of de-icing salts into the environment (e.g., yearly 17 million tons is applied in the USA, 6 million tons in Canada, while in Poland about 300 thousand tons) (Durickovic, 2020; General Directorate for National Roads and Motorways, 2020a, 2020b). Winter road de-icing is considered as substantial source of contamination (Amrhein & Strong, 1990; Hofman et al., 2012; Lindgren, 1996; Norrström & Bergstedt, 2001; Werkenthin et al., 2014). The peak of road de-icing occurs in winter and early spring (Hofman et al., 2012; Pająk et al., 2015). This phenomenon poses a serious threat to the soil and can be responsible for the disruption of ion-exchange relations, colloid dispersion, or the deterioration of soil structure (Robinson et al., 2017; Sun et al., 2015). Another important risk of de-icing is the effect of Na on heavy metal mobilization and their leaching to groundwater. Moreover, de-icing chemicals harm trees, causing symptoms such as decreased vitality and the loss of assimilative apparatus (Bäckström et al., 2004; Gałuszka et al., 2011).
Heavy metal chemistry strongly depends on the soil pH and on the spatial variation of soil organic matter (SOM) and clay minerals (Bradl, 2002; Kluge & Wessolek, 2012; Werkenthin et al., 2014; Zehetner et al., 2009). The major components of hummus capable of binding heavy metal cations (e.g., Pb2+, Zn2+, Mn2+, and Cu2+) are humic acid functional groups (mainly carboxylic, R–COOH) (Bradl, 2004). The pH-dependent character of the adsorption by SOM is an effect of the competition between hydrogen and other metals for exchange sites (Bradl, 2004; Peng et al., 2018; Weng et al., 2001). All these soil properties, responsible for heavy metal accumulation, may be modified by the influx of Na ions. Hence, changes in the heavy metal chemistry of adjacent forest soils are expected to be observed. Since roadways traverse a large area of forest soils, the aim of this study was to investigate an important effect of roadway vicinity on adsorption of metals, including selected heavy metals, to SOM. To clarify the complex effect of roads, particularly sodium input versus heavy metals, we studied in detail the geochemical modifications in these sites. We assessed properties of forest soils located up to 52 m from the forest edge. We hypothesized that (1) the concentration of heavy metals is modified by the influence of the amount of sodium originating from the road, but also by the variable contents of SOM, and therefore, (2) the vicinity of the roadway is a factor that modifies the concentration of heavy metals adsorbed to SOM in the adjacent forest soils.
2 Material and Methods
2.1 Study Site
Three study sites were established in the vicinity of the S7 express route (Table 1), between the cities of Kielce and Skarzysko-Kamienna, in central Poland (Fig. 1). The sites were located under single-species forest stands, where the dominant species is silver fir (Abies alba Mill.). In 2015, the average annual daily traffic for this S7 roadway section was 21,422 vehicles/day (vehicle types per day: motorcycle 58; car 15,757; van 1915; truck 3533; bus 159) (General Directorate for National Roads and Motorways, 2015). Forest stands in the investigated areas are multistoried and unevenly aged. Each site boarded the edge of the forest stand. The 15-m roadway verge is situated between the forest edge and the roadway.
The terrain at the study is relatively flat, with the elevation varying from 305 to 350 m above sea level. In accordance with the World Reference Base for Soil Resources (IUSS Working Group, 2014), the soils are classified as Albic Brunic Arenosol (site 1), Dystric Brunic Arenosol (site 2), and Dystric Cambisol (site 3). The soils are developed from two types of parent material: Cambrian sandstones and Triassic sandstones (Krajewski, 1955).
The area is characterized by an intermediate climate between the subcontinental and Central European (Woś, 1999). The average annual temperature in the years 1991–2005 was 7.8 °C, while the annual precipitation was on average 662 mm. The average number of days with snow cover is 70 days, while the length of the growing season is estimated at 210–220 days (Polish State Forests, 2011).
At each site, a set of 80 subsamples were collected according to the scheme in Fig. 1(c). Soil samples were taken from the organic (O) horizon and from the first 10-cm depth of the mineral (A) horizon. In total, 240 soil samples were taken (120 from the O horizon and 120 from the A horizon).
2.2 Laboratory Analysis
Live roots were first removed from the soil samples. The samples were then air-dried at room temperature for approximately 1 week and then sieved with a 2-mm mesh. Sieved samples were used to determine the particle size distribution using laser diffraction (Fritsch Analysette 22, Idar-Oberstein, Germany).
After 24 h of equilibration, the pH of soil suspended in distilled water (1:5 mass:volume) was measured using a potentiometric method with a combined electrode (Buurman et al., 1996).
To determine the sodium and heavy metal concentration, the samples were first mixed with 0.1 N spectrally pure HCl (10-g sample in 30 mL HCl) and shaken for 1 h. Using 0.1 N HCl allows to extract weakly bound to SOM fractions of metals—geochemically active exchangeable forms, which can be easily up-taken by plants (Kim et al., 2002; Wei et al., 2005). These extraction mixtures were then filtered and rinsed with the extractant to obtain a volume of 100 mL. The concentrations of Cu, Ni, Pb, Zn, and Na were then determined by ICP-OES (inductively coupled plasma optical emission spectrometry) with iCAP6000 Thermo (Thermo Fisher Scientific, Waltham, MA, USA). Each sample was measured with two replicates, with acceptable difference between the measurements < 10%. The quality control (QC) was performed using single-element aqueous certified reference materials (CRM): Na (No. 1038021-29, LGC) 1005 ± 5 μg μL−1; Cu (No. M00390, Agilent) 1001 ± 2 μg mL−1; Ni (No. M00866, Agilent) 1000 ± 2 μg mL−1; Pb (No. M00643, Agilent) 1001 ± 2 μg mL−1; Zn (No. J00510, Agilent) 1001 ± 2 μg mL−1. The QC was performed at the beginning and the end of each experimental run, as well as after each 10 measurements.
Subsamples were ground by a ball mill (Fritsch) to obtain heterogeneity. These fine subsamples were used to measure the total carbon (C) and nitrogen (N) concentrations with a LECO CNS TrueMacAnalyser (Leco, St. Joseph, MI, USA). The quality control of C and N measurements were assured by analysis of Organic Analytical Standards (OAS, Elemental Microanalysis Ltd.) Nos. B1280 (C = 0.83 ± 0.05%, N = 0.07 ± 0.01%); B2178 (C = 2.46 ± 0.22%, N = 0.16 ± 0.01%); and AR4021 (C = 3.04 ± 0.16%). Each standard and sample were measured with two replicates, with acceptable difference between the measurements < 10%. Analysis of standards was performed at the beginning and the end of each experimental run. The limit of quantitation was 0.008% for C and 0.266% for N. All soil samples were carbonate-free; therefore, C was considered to be organic carbon.
2.3 Statistical Analyses
The data set was divided into groups according to the soil horizon and site for statistical analysis (the mean and the standard error were calculated). The soil samples were also divided into groups according to the distance from the road. The Kolmogorov-Smirnov (K-S) and Tukey tests were performed to assess differences in the metal concentrations between the groups. The Pearson correlation coefficients of the investigated soil properties were calculated, and principal component analysis (PCA) was conducted. Significance was defined at p < 0.05 unless otherwise specified. The statistical analyses were carried out using R statistical software (R Core Team, 2020) and Statistica 13 (Dell Inc., 2016).
3 Results
The particle size distribution of the investigated soils differed significantly between sites. Site 1 soils were sandy (on average: sand 72%, silt 24%, and clay 4%), whereas site 2 contained silty soil (sand 43%, silt 49%, clay 8%), as well as site 3 (sand 31%, silt 62%, and clay 7%). Given these differences, the investigated soil properties shown in Table 2 are displayed as site 1, site 2, and site 3.
The O horizon of site 1 had significantly lower organic C and N concentrations than site 2. In the horizon A, significantly lower C and N concentrations were found in site 2 in comparison to site 1 and site 3 (Table 2). The pH value of investigated sites was over 4, except for the A horizon in site 3. There was a pattern that HCl-extractable heavy metals in O horizon had higher concentrations than in A horizon. Some significant differences between the sites were found. Increased Cu concentration in the O horizon and lowered in the A horizon of site 2 are clearly noticeable. All sites did not differ significantly in Zn and Cu concentration in the O horizon.
To investigate the influence of road proximity on the soil Na concentration, we compared the Na concentrations of the distance groups using the ANOVA and Tukey test. In all sites, the Na concentration in the O and A horizons was significantly higher in samples located 2–7 m from the forest edge than in samples taken further from the forest edge (12–52 m), with p < 0.01.
We merged the data from sites 1, 2, and 3 for further analysis, according to the soil horizons. Given the spatial variation of the HCl-extractable Na concentration (Fig. 2), and results of Tukey test, we divided the samples into two groups according to distance from the forest edge, 2–7 m and 12–52 m. The purpose of such grouping was to test the sudden concentration shifts with the distance. The results are shown in Table 3. An analysis of the stoichiometry (metal to C [mg:kg] ratio) was also performed to standardize the concentrations of the investigated metals to variable C concentration. For each sample, we calculated the ratio between a given heavy metal and C. This standardization allows for meaningful comparisons of metal concentration, especially when associated with soil organic matter.
The C and N concentrations did not significantly differ between the distance groups in the O horizon, while differences were found in the A horizon, revealing higher concentrations closer to the roadway (Table 3). In the O horizon, the pH was significantly elevated near the roadway. The Na:C ratio was significantly increased in close proximity to the roadway in both horizons. Higher Na:C ratios were found in the A horizon than in O horizon. The concentration of HCl-extracted Ni was increased in close proximity to the road in the A horizon, while significant differences in the Ni:C ratio were not found for either horizon. The Pb concentration in the O horizon was significantly depleted at the 2–7-m distance, as was the Pb:C ratio in A horizon. We also found significantly increased concentrations of Zn in A horizon and a significantly increased Zn:C ratio in the O horizon. The K-S test revealed no significant differences in the Cu concentration and Cu:C ratio between the paired groups.
Carbon and nitrogen were both negatively correlated with the pH in the O horizon (r = − 0.44 and r = − 0.43, respectively). The soil pH was also negatively correlated with the Pb concentration in both horizons (r = − 0.67 in the O horizon, r = − 0.45 in the A horizon). The C and N concentrations were significantly positively correlated with all investigated heavy metals in both horizons. In O horizon, Na was significantly correlated only with Zn (r = 0.31), while in A horizon Na was significantly correlated with all analyzed heavy metals (Cu: r = − 0.39; Ni: r = 0.42; Pb: r = 0.22; Zn: r = 0.44).
The analysis of relationships between the HCl-extractable heavy metal concentrations (Fig. 3) and their stoichiometric ratios to C plotted against pH (Fig. 4) revealed that the majority (11 of 16 tests) of analyzed linear regressions were statistically significant. The regression parameters are given in the Appendix. Cu in the O horizon, as well as Cu:C ratio in both horizons, did not correspond with pH. Similarly, no relations were found for the pH and Ni in the O horizon, as well as Pb:C ratio in the A horizon. Generally, regression models for metal:C ratios explained more variance (Fig. 4).
We performed principal component analysis (PCA) to investigate relationships between the metal:carbon ratios, soil pH, C concentration, and clay content. Owing to the lack of data, the clay content for the O horizon is from the underlying mineral. Together, factors 1 and 2 used in the PCA explained 68.6% and 67.2% of the variance for O and A horizons, respectively. Similar relationships between analyzed variables were found for both horizons (Fig. 5a, b). The ratios of Ni and Zn to C were mostly related to soil pH, while the ratios of Pb and Cu to C were more related to the clay content. These relationships with the clay content were stronger in the A horizon.
4 Discussion
HCl extraction reveals only the fraction of total metals in the soil. There is little information in the literature about the geochemical background of HCl-extractable metals and their relationship to the total concentration. To estimate the fraction of HCl-extractable metals in their total form, we used previously obtained data from site 3, established on Dystric Cambisol (Firek, 2020, thesis), where the total forms of Cu, Ni, Pb, and Zn were measured by TXRF (total X-ray fluorescence) spectrometry. This work provided the following values (mean ± standard error, n = 40): Cu 10.49 ± 0.69 mg kg−1; Ni 8.67 ± 2.80 mg kg−1; Pb 99.46 ± 5.41 mg kg−1; and Zn 96.96 ± 9.73 mg kg−1. According to the guidelines for assessing the degree of heavy metal contamination in soil, the obtained data indicate low levels of Pb pollution (taking 50 mg kg−1 as the upper limit for the natural background) (Kabata-Pendias & Pendias, 1993). The increased Pb concentration may be related to the historical use of Pb-containing fuels; alternatively, it might be related to the geochemical background. The average (n = 40) total fractions of HCl-extractable metals in the O horizon were Cu 46%, Ni 23%, Pb 48%, and Zn 34%; in the A horizon, these were Cu 20%, Ni 5%, Pb 22%, and Zn 5%. Thus, the fraction of HCl-extractable metal depends on the horizon, specifically to the concentrations of organic matter and metals (Pb > Cu > Zn > Ni).
Based on the data presented in Table 2, the significant differences in the concentrations of HCl-extractable Pb, Cu, Zn, and Ni between the analyzed sites appear to be related to the different mineral compositions. The sites differed in both the parent material and the soil unit. Sites 1 (Albic Brunic Arenosol) and 2 (Dystric Brunic Arenosol) were located on Triassic sandstone and site 3 (Dystric Cambisol) was located on Cambrian sandstone.
Soil samples in close proximity to the S7 roadway had significantly increased Na concentrations. The data in Fig. 2 and Table 3 indicate that there are zones that differ in Na concentration, both in the O and A horizons. The most probable source of Na input close to the road (up to 10 m from the forest edge) is the process of winter road de-icing, in which NaCl is primarily applied (Willmert et al., 2018). The zone of significant Na influence observed in this research is estimated to cover approximately 25 m, including the distance between the roadway and the forest edge (approximately 15 m). This identified Na impact zone is larger than in previous related studies. For example, Hofman et al. (2012) found that the Na impact zone was usually limited to 10 m from the road edge. Similarly, other studies (e.g., Lundmark & Olofsson, 2007; Zehetner et al., 2009) found that the roadway has a minimal effect at distances greater than 10 m from the road edge. The scale of roadway impact on forest soils is strongly dependent on many factors, such as the traffic volume, wind, roadside vegetation, soil texture, distance from the road, slope topography, and structure of forest stand boundary (Bryson & Barker, 2002). The influence of Na on the geochemical background of soils in the zone 2–7 m from the forest edge was significant. It has been reported that the Na concentration in the soil near roadways is highest during winter or at the beginning of spring (Asensio et al., 2017; Bäckström et al., 2004; Pająk et al., 2015).
The significant inflow of Na ions increased the pH of the O horizon in the vicinity of the road (Table 3). An increased sodium concentration results in soil alkalization (Bryson & Barker, 2002), which leads to the release of H+ from SOM functional groups and their diffusion into the soil solution (Shanley, 1994). This soil chemistry modification increases the potential of SOM to bind heavy metals owing to an increased number of available exchange sites. This phenomenon is strictly related to the soil solution pH (Weng et al., 2001).
The Pb concentration in soils near the roadway was not increased. Indeed, the Pb concentration in soils near the road was significantly lower than at further distances, but only in the O horizon. Similarly, the Pb:C ratio near the roadway was lower in A horizon, suggesting that Pb is not currently supplied in considerable amounts from road traffic. The concentrations of Pb and the Pb:C ratio were negatively correlated with pH; together with principal component analysis (Fig. 5a, b), this implied an affinity of Pb for clay minerals rather than for SOM.
Sipos et al. (2005) concluded that increased soil pH, which is affected by carbonates, increases Pb transport into deeper soil layers. Our results indicate that Na ions can also be regarded as a significant alkalizing factor that increases Pb leaching. Although soil organic matter plays a major role in Pb adsorption, binding to clay minerals is nevertheless a stronger process (Sipos et al., 2005). The obtained results also corresponded to the findings of Bäckström et al. (2004), who identified a correlation between heavy metal concentrations and the pH in roadside soil; this correlation was strengthened in winter when de-icing agents were applied. These authors found depleted Pb concentrations in soils near the roadway.
The Zn concentration was significantly greater near the road in A horizon, but the Zn:C ratio was higher near the road only in the O horizon. Simple regression models of Zn and Zn:C to pH revealed significant relationships (especially between Zn:C and pH in the O horizon, Fig. 4). Moreover, PCA confirmed the pH-dependent character of Zn accumulation, competing with H+ for exchange sites of SOM. Our findings are consistent with kinetic models of Zn solubility, confirming that SOM is the major soil element influencing Zn sorption (Peng et al., 2018). A similar pattern was found for Ni. The Ni concentration was higher near the roadway in the A horizon (Table 3). The PCA also provided evidence for the pH dependency of Ni accumulation in soil. Increased concentrations of Ni and Zn owing to the vicinity of the roadway were previously found by Bäckström et al. (2004) and Zehetner et al. (2009). These studies also confirmed that Ni and Zn accumulation/mobility are strongly pH dependent. An increased pH creates available exchange sites that adsorb Ni and Zn ions, thereby immobilizing these elements. This also results in the apparent increase of Ni and Zn concentrations in soils near roads. Such a mechanism was also suggested by Bradl (2004).
Unlike Ni and Zn, the concentrations of Cu were not increased near the road, nor were the Cu:C ratios in both horizons (Table 3). Furthermore, there was no significant dependency between Cu or Cu:C on the pH (Figs. 3 and 4). SOM is considered to be the major soil component that binds Cu (Kabata-Pendias & Pendias, 1993). Cu adsorption/desorption has been identified as pH dependent (Bäckström et al., 2004). Also properties like dissolved organic carbon concentration in drainage waters, as well as total forms of metal, play a significant role in Cu solubility (Tipping et al., 2003). However, our PCA revealed a stronger correlation between Cu and the clay content (Fig. 5a, b). The importance of the clay fraction as the most reactive soil fraction for Cu binding was previously identified by Shi et al. (2020).
5 Conclusions
Our investigation suggests that the S7 roadway influences the topsoil chemistry of the adjacent forest soils up to a distance of approximately 10 m from the forest edge. The most obvious effect was the increased Na concentration, which originated from winter road de-icing. The effect of road proximity on the heavy metal concentration in soil was relatively weak. The data analysis implied that the effect of the road on the accumulation of heavy metals (Cu, Ni, Pb, Zn) was indirect. As a mechanism, we suggest that Na increases the soil pH, leading to the release of H+ ions from SOM functional groups, thereby enhancing the ability of SOM to bind heavy metals (especially Ni and Zn). The concentrations of HCl-extractable Ni and Zn, as well as the Ni:C and Zn:C stoichiometric ratios, were associated with pH. The HCl-extractable Ni concentration in the A horizon was higher in proximity to the roadway, revealing its strong pH dependency. Higher values near the roadway and pH dependency were also found for Zn in both the O and A horizons, with an increased pH close to the road. Pb and Cu ions, as well as the Pb:C and Cu:C ratios, indicated a stronger affinity of these metals for binding to clay minerals. Pb was also depleted in the area near the road, which was likely because of strong Na ion competition.
Data Availability
The dataset analyzed during the current study is not publicly available due to the corresponding author does not possess full rights to the entire dataset, but it may be available on a reasonable request.
References
Amrhein, C., & Strong, J. E. (1990). The effect of deicing salts on trace metal mobility in roadside soils. Journal of Environmental Quality. https://doi.org/10.2134/jeq1990.00472425001900040022x.
Asensio, E., Ferreira, V. J., Gil, G., García-Armingol, T., López-Sabirón, A. M., & Ferreira, G. (2017). Accumulation of de-icing salt and leaching in Spanish soils surrounding roadways. International Journal of Environmental Research and Public Health. https://doi.org/10.3390/ijerph14121498.
Bäckström, M., Bäckman, L., Folkeson, L., Karlsson, S., & Lind, B. (2004). Mobilisation of heavy metals by deicing salts in a roadside environment. Water Research. https://doi.org/10.1016/j.watres.2003.11.006.
Bradl, H. (2002). Adsorption of heavy metal ions on clays. In A. Hubbard (Ed.), Encyclopedia of surface and colloid science. Volume (p. 373). Dekker.
Bradl, H. B. (2004). Adsorption of heavy metal ions on soils and soils constituents. Journal of Colloid and Interface Science. https://doi.org/10.1016/j.jcis.2004.04.005.
Bryson, G. M., & Barker, A. V. (2002). Sodium accumulation in soils and plants along Massachusetts roadsides. Communications in Soil Science and Plant Analysis. https://doi.org/10.1081/CSS-120002378.
Buurman, P., van Lagen, B., & Velthorst, E. J. (1996). Manual for soil and water analysis. Backhuys Publishers.
Chrzan, A. (2013). Zawartość wybranych metali ciężkich w glebie i faunie glebowej. Proceedings of ECOpole. https://doi.org/10.2429/proc.2013.7(2)072.
Councell, T. B., Duckenfield, K. U., Landa, E. R., & Callender, E. (2004). Tire-wear particles as a source of zinc to the environment. Environmental Science & Technology. https://doi.org/10.1021/es034631f.
Krajewski, R. (1955) Detailed Geological Map of Poland. Polish Geological Institute, Warsaw.
Durickovic, I. (2020). NaCl material for winter maintenance and its environmental effect. Salt in the Earth, 1–19. https://doi.org/10.5772/intechopen.86907.
IUSS Working Groups WRB (2014). World reference base for soil resources. World Soil Resources Report 106, FAO, Rome.
Gałuszka, A., Migaszewski, Z. M., Podlaski, R., Dołęgowska, S., & Michalik, A. (2011). The influence of chloride deicers on mineral nutrition and the health status of roadside trees in the city of Kielce, Poland. Environmental Monitoring and Assessment. https://doi.org/10.1007/s10661-010-1596-z.
General Directorate for National Roads and Motorways (2015). Average annual daily traffic (AADT) at measuring points in 2015 on state roads. https://www.gddkia.gov.pl/userfiles/articles/g/generalny-pomiar-ruchu-w-2015_15598//SYNTEZA/WYNIKI_GPR2015_DK.pdf. Accessed 03 February 2021.
General Directorate for National Roads and Motorways. (2020a). We’ll recap the winter of 2019/2020 and explain what road maintenance is all about (in Polish). https://www.gddkia.gov.pl/pl/a/37500/Podsumujemy-zime-20192020-i-wyjasnimy-o-co-chodzi-w-utrzymaniu-drog?fbclid=IwAR3ONfRET5Kr-Lrbkp6hVLmDlyu5G2pHU30_MA2FYQxv-0muX8filn8ninQ. Accessed 08 April 2021.
General Directorate for National Roads and Motorways. (2020b). Ready for all weather. The 2020/2021 winter season has begun (in Polish). https://www.gddkia.gov.pl/pl/3013/Zimowe-utrzymanie-drog. Accessed 08 April 2021.
Grigalaviciene, I., Rutkoviene, V., & Marozas, V. (2005). The accumulation of heavy metals Pb, Cu and Cd at roadside forest soil. Polish Journal of Environmental Studies, 14, 109–115.
Hofman, J., Trávníčková, E., & Anděl, P. (2012). Road salts effects on soil chemical and microbial properties at grassland and forest site in protected natural areas. Plant, Soil and Environment, 58, 282–288.
Kabata-Pendias, A., & Pendias, H. (1993). Biogeochemistry of trace metals (in Polish). Polish Scientific Publishers PWN.
Kelly, J., Thornton, I., & Simpson, P. R. (1996). Urban geochemistry: A study of the influence of anthropogenic activity on the heavy metal content of soils in traditionally industrial and non-industrial areas of Britain. Applied Geochemistry. https://doi.org/10.1016/0883-2927(95)00084-4.
Kim, S.-O., Kim, K.-W., & Stüben, D. (2002). Evaluation of electrokinetic removal of heavy metals from tailing soils. Journal of Environmental Engineering. https://doi.org/10.1061/(ASCE)0733-9372(2002)128:8(705.
Kluge, B., & Wessolek, G. (2012). Heavy metal pattern and solute concentration in soils along the oldest highway of the world—The AVUS Autobahn. Environmental Monitoring and Assessment. https://doi.org/10.1007/s10661-011-2433-8.
Kowalik, P. (2001). Protection of the soil environment (in Polish). Polish Scientific Publishers PWN.
Laxen, D. P. H., & Harrison, R. M. (1977). The highway as a source of water pollution: An appraisal with the heavy metal lead. Water Research. https://doi.org/10.1016/0043-1354(77)90175-0.
Lindgren, Å. (1996). Asphalt wear and pollution transport. Science of the Total Environment. https://doi.org/10.1016/0048-9697(96)05220-5.
Lundmark, A., & Olofsson, B. (2007). Chloride deposition and distribution in soils along a deiced highway—Assessment using different methods of measurement. Water, Air, & Soil Pollution. https://doi.org/10.1007/s11270-006-9330-8.
Motto, H. L., Daines, R. H., Chilko, D. M., & Motto, C. K. (1970). Lead in soils and plants: Its relationship to traffic volume and proximity to highways: discussions follow. Environmental Science & Technology. https://doi.org/10.1021/es60038a009.
Norrström, A. C., & Bergstedt, E. (2001). The impact of road de-icing salts (NaCl) on colloid dispersion and base cation pools in roadside soils. Water, Air, & Soil Pollution. https://doi.org/10.1023/A:1005221314856.
Nriagu, J. O., & Pacyna, J. M. (1988). Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature. https://doi.org/10.1038/333134a0.
Pająk, M., Krzaklewski, W., Duda, K., & Gruba, P. (2015). Spatial and temporal variation in soil salinity as a result of chemical de-icing of road in Krakow, Poland. Fresenius Environmental Bulletin, 24, 3363–3370.
Peng, L., Shi, Z., Wang, P., Li, W., Lin, Z., Dang, Z., & Sparks, D. L. (2018). A novel multi-reaction model for kinetics of Zn release from soils: Roles of soil binding sites. Journal of Colloid and Interface Science. https://doi.org/10.1016/j.jcis.2017.12.006.
Polish State Forests. (2011). Nature and forest conditions of Suchedniów Forest Inspectorate (in Polish). A report. https://suchedniow.radom.lasy.gov.pl/documents/11058/18775352/warunki+przyrodniczo-lesne.pdf. Accessed 03 February 2021.
R Core Team (2020). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing, https://www.R-project.org/. Accessed 03 Frbeuary 2021.
Reimann, C., & de Caritat, P. (2005). Distinguishing between natural and anthropogenic sources for elements in the environment: Regional geochemical surveys versus enrichment factors. Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2004.06.011.
Robinson, H. K., Hasenmueller, E. A., & Chambers, L. G. (2017). Soil as a reservoir for road salt retention leading to its gradual release to groundwater. Applied Geochemistry. https://doi.org/10.1016/j.apgeochem.2017.01.018.
Shanley, J. B. (1994). Effects of ion exchange on stream solute fluxes in a basin receiving highway deicing salts. Journal of Environmental Quality. https://doi.org/10.2134/jeq1994.00472425002300050019x.
Shi, Z., Yang, Y., Liu, F., Lu, Y., Ye, Q., Lin, X., Feng, C., Liang, Y., & Dang, Z. (2020). Effect of soil particle size on the kinetics of Cu release from field-contaminated soils: Experiments and a quantitative model. Chemical Geology. https://doi.org/10.1016/j.chemgeo.2020.119780.
Sipos, P., Németh, T., & Mohai, I. (2005). Distribution and possible immobilization of lead in a forest soil (Luvisol) profile. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-004-1581-y.
Soleimani, M., Amini, N., Sadeghian, B., Wang, D., & Fang, L. (2018). Heavy metals and their source identification in particulate matter (PM2.5) in Isfahan City, Iran. Journal of Environmental Sciences. https://doi.org/10.1016/j.jes.2018.01.002.
Spellberg, I. F. (1998). Ecological effects of roads and traffic: A literature review. Global Ecology and Biogeography, 7, 317–333.
Sun, H., Alexander, J., Gove, B., & Koch, M. (2015). Mobilization of arsenic, lead, and mercury under conditions of sea water intrusion and road deicing salt application. Journal of Contaminant Hydrology. https://doi.org/10.1016/j.jconhyd.2015.07.002.
Tipping, E., Rieuwerts, J., Pan, G., Ashmore, M. R., Lofts, S., Hill, M. T. R., et al. (2003). The solid-solution partitioning of heavy metals (Cu, Zn, Cd, Pb) in upland soils of England and Wales. Environmental Pollution, 125(2), 213–225. https://doi.org/10.1016/S0269-7491(03)00058-7.
Tossavainen, M., & Forssberg, E. (1999). The potential leachability from natural road construction materials. Science of the Total Environment. https://doi.org/10.1016/S0048-9697(99)00283-1.
Viard, B., Pihan, F., Promeyrat, S., & Pihan, J. C. (2004). Integrated assessment of heavy metal (Pb, Zn, Cd) highway pollution: Bioaccumulation in soil, Graminaceae and land snails. Chemosphere. https://doi.org/10.1016/j.chemosphere.2004.01.003.
Ward, N. I., Brooks, R. R., & Reeves, R. D. (1974). Effect of lead from motor-vehicle exhausts on trees along a major thoroughfare in Palmerston North, New Zealand. Environmental Pollution. https://doi.org/10.1016/0013-9327(74)90031-7.
Warren, R. S., & Birch, P. (1987). Heavy metal levels in atmospheric particulates, roadside dust and soil along a major urban highway. Science of the Total Environment. https://doi.org/10.1016/B978-012424210-4/50010-3.
Wei, Y.-L., Yang, Y.-W., & Lee, J.-F. (2005). Lead speciation in 0.1N HCl-extracted residue of analog of Pb-contaminated soil. Journal of Electron Spectroscopy and Related Phenomena. https://doi.org/10.1016/j.elspec.2005.01.218.
Weng, L., Temminghoff, E. J. M., & Van Riemsdijk, W. H. (2001). Contribution of individual sorbents to the control of heavy metal activity in sandy soil. Environmental Science & Technology. https://doi.org/10.1021/es010085j.
Werkenthin, M., Kluge, B., & Wessolek, G. (2014). Metals in European roadside soils and soil solution—A review. Environmental Pollution. https://doi.org/10.1016/j.envpol.2014.02.025.
Willmert, H. M., Osso, J. D., Twiss, M. R., & Langen, T. A. (2018). Winter road management effects on roadside soil and vegetation along a mountain pass in the Adirondack Park, New York, USA. Journal of Environmental Management. https://doi.org/10.1016/j.jenvman.2018.07.085.
Wong, C. S. C., Li, X., & Thornton, I. (2006). Urban environmental geochemistry of trace metals. Environmental Pollution. https://doi.org/10.1016/j.envpol.2005.09.004.
Woś, A. (1999). The climate of Poland (in Polish). Polish Scientific Publishers PWN.
Zehetner, F., Rosenfellner, U., Mentler, A., & Gerzabek, M. H. (2009). Distribution of road salt residues, heavy metals and polycyclic aromatic hydrocarbons across a highway-forest interface. Water, Air, & Soil Pollution. https://doi.org/10.1007/s11270-008-9831-8.
Zereini, F., Alt, F., Messerschmidt, J., Wiseman, C., Feldmann, I., Von Bohlen, A., Müller, J., Liebl, K., & Püttmann, W. (2005). Concentration and distribution of heavy metals in urban airborne particulate matter in Frankfurt am Main, Germany. Environmental Science & Technology. https://doi.org/10.1021/es040040t.
Acknowledgements
We thank Bożena Dobroś, Regina Głowacka, and Agnieszka Wojciechowicz for their help with chemical analysis. We also thank Katherine Thieltges, from Edanz Group (https://en-author-services.edanzgroup.com/ac), for editing a draft of this manuscript. The authors are grateful to an anonymous reviewer for his helpful suggestions and comments, which considerably improved the manuscript.
Code Availability
Not applicable.
Funding
This work was supported by the National Science Centre, Poland, agreement no. UMO-2017/25/B/ST10/02128.
Author information
Authors and Affiliations
Contributions
DK: conceptualization, investigation, writing—original draft, visualization, validation; MK: investigation, writing—original draft; MP: visualization, investigation; AŁ: investigation, writing—review and editing; PG: conceptualization, writing—review and editing, funding acquisition
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kupka, D., Kania, M., Pietrzykowski, M. et al. Multiple Factors Influence the Accumulation of Heavy Metals (Cu, Pb, Ni, Zn) in Forest Soils in the Vicinity of Roadways. Water Air Soil Pollut 232, 194 (2021). https://doi.org/10.1007/s11270-021-05147-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-05147-7