Abstract
Successful drilling operations are dependent on the properties of the drilling fluid used to drill wells. Barite is used as a weighting agent during the preparation of drilling fluid. Over the years, oil and gas industry in Nigeria has been depending mainly on imported barite for drilling operations, whereas the country has huge deposits of barite. There is the need to assess the properties of the locally sourced barite for their suitability in drilling fluid formulation. This study presents the local processing methods of barite and examines the crude and on-the-site processed barite’s physio-chemical properties. These parameters were compared with American Petroleum Institute and Department of Petroleum Resources standards. XRD results show that on-the-site beneficiated barite has 87.79% BaSO4, 6.66% silica, 0.03% total soluble salt, 1.39% Fe2O3, and 1.603% heavy metals. Chemical analysis indicated that the pH, moisture content, metallic content such as Ca, Pb, Zn, Mg, Cu, and Cd minerals, and extractable carbonates were within the standard specified for usage as a drilling fluid weighting agent. The analysed crude barite samples were basic, within the pH of 8.3 and 8.6. Locally processed barite has lower Fe, Pb, Cd, and Cu content compared to industrially accepted barite. The specific gravity increased from 4.02 ± 0.07 to 4.15 ± 0.13, and the hardness reduced potentially from 5 Mohr to 3.5 Mohr on the hardness scale. The amount of impurities was sufficiently low, and the specific gravity of the samples improved to meet the needs of any drilling operation and compare favourably with industrially accepted barite.
Similar content being viewed by others
Introduction
Over 80% of barite produced worldwide is used as a weighting agent in drilling fluids. World barite resources are about 2 billion tons, but only 740 million metric tons have been identified. China, India, Kazakhstan, Morocco, Thailand, USA, Turkey and other countries produced 9.5 million metric tons in 2018 which worth 2688 million US dollars. Brown barite, residual deposit type barite, barite 4.3 and oil and gas industry barite market generated revenue of 806.4 million US dollars, 860.2 million US dollars, 645.1 US dollars and 564.5 US dollars, respectively. Barite is used for drilling and other industrial application around the world (Garside 2020; Research Drive 2020; USGS 2017, 2019). Nigeria in Africa, currently holds the fourth largest deposits of barite in the world with an estimated reserve of over 21 million metric tons. Ironically, the nation is not anywhere in the list of globally recognized largest producers USGS of barite for drilling activities and other industrial applications (Garside 2020; USGS 2020). Principal factors responsible for this include the unregulated importation of unprocessed barite ore, limited access to mining site and high-quality barite ore grade, the absence of mechanized mining companies, unregulated artisanal and small-scale mining (ASM) activities, and lack of industry-institution-government collaboration among the oil and gas industries, research institutes and local barite producers (MMSD 2010; NGSA 2010).
There are low-, medium- and high-quality barite in Nigeria as shown in Table 1 and Fig. 1 (Essalhi et al. 2018; Oden 2012). The mining of barite between 0 and 5 m depth gave rise to low-quality barite due to the presence of associated impurities and low-pressurized hydrothermal fluids that are deficient of BaSO4. However, further studies on some barite deposits have shown that mining of barite deposits below 15 m depth produces medium- to high-quality grades of barite (Afolayan 2017; Inyang et al. 2013; MMSD 2016; Oden 2012). In addition, some notable research and exploration studies report on the low, medium, and high grades with the recommendation that further processing is done to evaluate the potential of few identified high-grade deposits (Chaanda et al. 2010; Labe et al. 2018; Mgbemere et al. 2018a, b; Oden 2012). Barite ore requires some upgrading to minimum purity, specific gravity, and composition with qualities needed in drilling and other industrial activities (Ibrahim et al. 2017; MMSD 2010; NGSA 2010). This can be achieved at a reduced cost when beneficiation is done at or close to the mining site using basic and efficient processing methods (API 2004, 2010; Tanko et al. 2015). However, barite’s on-site beneficiation is a pre-processing approach and is not sufficient to upgrade low quality barite ore to the American Petroleum Institute (API) standard.
The demand for high-quality barite as a drilling fluid weighting agent has been increasingly difficult to meet due to variation in the level and form of gangue minerals in the ore. The operations of local barite processors, and the use of gravity separation, chemical leaching, and flotation techniques to upgrade barite quality have been explored and reported in the literature (Achusim-Udenko et al. 2011; Chen et al. 2019a, b; Deng et al. 2019; Grigorova et al. 2015; Liu et al. 2019; Mgbemere et al. 2018a, b; Nzeh and Hassan 2017; Raju et al. 2016; Wang et al. 2014; Zhao et al. 2014). However, these reports do not show much evidence on specific methods for evaluation or performance of local processing methods of barite in Nigeria so as to reveal the local product’s quality and present a parallel comparison between the industrially accepted barite imported from Morocco and local products. The environmental concern posed by flotation and other adhering chemicals and the elimination of water-soluble salts of Na, K, Ma, and Ca in barite due to acidic leaching were not considered.
Field experiments of small-scale processing that reflects the practice of barite processing locally need to be done as a way of leveraging on the laboratory-based experiments as shown in previous studies in Table 2 to translate theory into practice. Furthermore, the requirements or standards for weighting agents in drilling mud exceed just specific gravity need, which were the major focus of research works as presented in Table 2. There are physio-chemical properties that determine the rheological properties of the drilling mud. Predominant surface mining of barite indeed contributed to the low-quality stigma of mined products in Nigeria. However, it is essential to show the extent to which the local product is inferior or superior to the industrially accepted barite imported from Morocco and to indicate steps that should be taken to reverse the trend of excessive importation of barites.
Barite importation in Nigeria represents 3.6% of total industrial minerals importation with an estimated consumption of 440,000 metric tons for 2020 and valued at 96 million US dollars. Although there is an existing policy on the ban of barite importation since 2011, the government cannot effectively enforce this until the local barite supply chain can guarantee sustainable supply of the commodity. However, to close the demand–supply gap, the Nigerian government has taken some steps to reverse importation (MMSD 2016, 2020). These steps can only succeed if scientific data and information enable informed decisions to regulate stakeholders along the barite value chain. Efforts include that of Raw Materials Research and Development Council, Nigerian Local Content Monitoring Board, Nigerian Geological Survey Agency, Federal Ministry of Mines and Steel Development; activities by the private sector such as Barites Miners, and Processors association, TOTAL Plc, etc. The present work is designed to give scientific support to the efforts.
In this research, the local processing of barite was presented. The physio-chemical properties of the crude and on-the-site processed barite were measured and compared with imported barite and API standards. The locally processed barite was examined for its suitability as a weighting agent additive in drilling fluid. Recommendations were made to develop and optimize processing methods that will enhance the quality of locally processed barite in order to compete favourably with barite products in the global market used for different drilling activities in the oil and gas industry. The manuscript, in a nutshell, seeks to provide relevant scientific information to support efforts of some government agencies and the joint committee on barite processors-oil producing companies in Africa on the value addition of barite deposits in the Middle Benue Trough of Nigeria.
Research methods
Location, mapping, and evaluation of Torkula barite deposit
The Benue barite reserves consist of Lessel barite and Guma barite fields. Lessel field in Benue State has large number of veins and occur mainly in the south of Gboko (all through the Yandev deposit North of Gboko), Lessel Bunde, Lessel Mbato and Lessel Mbagwa. Currently, there are more than 20 veins in production. Similarly, the Guma field is made up of vein deposits in Iye, Kaseyo, Zanzan, Makurdi, and Torkula–Hungwa axis. The highest concentration of barite veins in the field is within the Torkula–Hungwa axis. Although the number of known veins in the axis is currently fewer than in Azara, and Kumar, its barite deposits holds a lot of promise. Recent field work at three of the veins revealed that the third vein is massive and has produced thousands of tons of barite with specific gravity above 4.2 (Afolayan 2017; Oden 2012). This is presented in Table 1. Aside the fieldwork, there are little to no research work done on barite deposits within Torkula–Hungwa axis other than the mineral mapping carried out by the Nigeria Geological Survey agency on behalf of the Polyguard Investment Nigeria, Ltd (Afolayan 2017).
Torkula barite deposit is located in Guma local government area in Benue State of Nigeria and has a total land mass of about 28,882 km2 as shown in Fig. 2. It is surrounded with sedimentary rocks and the soil is dominantly associated with mineral deposits like lead, salt, barite, feldspar, and brown earth of volcanic origin. Guma lies between latitude N7 32 and N8 51 and longitude E9 35 and E9 22 with an average high rainfall of about 1198–1798 mm annually. Relative humidity is between 43 and 86%, temperature ranges from 27 and 37 °C (Sombo et al. 2016). The highest concentration of barite veins in this field is within and around Torkula because of its size. However, there is barite quality contrast between the top part and the lower portions of the vein. This disparity is due to major impurities such as quartz, iron oxide (goethite), fluorite, and carbonates of iron, calcium and magnesium. (API) (API 2004, 2010; Fatoye et al. 2014; Oden 2012).
Sampling and on-the-site beneficiation of barite
The mining of barite in Torkula barite field is done on a large scale. Soil overburden deposits are removed from the surface of barite veins and explosives are used for the fragmentation of the barite rocks. With the aid of an excavator and a loader, the blasted run-of-mine (barite ore) is removed from the pit of about 35 m deep and loaded into a truck. Heaps of barite ore mixed with impurities. Also, local processing (on-site beneficiation) and screening of processed barite at Torkula–Hungwa barite field is performed. Barite and non-barite minerals in the barite ore are separated under the influence of gravity. This was done by applying pressurized water, pumped from the mined pit on the barite ore in a 2 m by 2 m wooden box. However, the pump pressure is reduced at the upgrading stages to allow complete sedimentation of minerals based on specific gravity difference. As the water flows over the barite ore, barite and non-barite minerals are separated/handpicked and kept in separate bags.
Barite samples were randomly collected from the heaps of barite run-of-mine. White barite was separated from the brownish, reddish and other coloured barite mineral (this is believed to contain non-barite minerals), collected into cellophane bags (black polythene bag) and appropriately labelled. Since the heaps have been separated, it is believed that the materials from each heaps are from similar depths. Collected samples were categorized into solid and milled samples. Similarly, the solid samples were labelled as solid samples A, B, C, D, E and F. On the other hand, milled or pulverized samples were labelled as unprocessed samples (Un. PS) A, B, C, D and on-site processed sample (On-SPS) as shown in Fig. 3. Solid sample A is white-coloured barite with relatively small portion of sandstones, brown-coloured barite was labelled solid sample B, different fractions of white and transparent barite samples as solid samples C and D. However, solid samples E and F were whitish with some associated gangue minerals interlocked within the barite vein. For a comprehensive comparison and scientific criticism, research works on reference barite samples collected from Nigeria Petroleum Development Company (NPDC) Warri Field Office were cited. The air-tight barite samples selected from the Hungwa mining site were crushed and milled using Jaw crusher combined with pendulum pulverized milling machine.
Sample preparation
The crushed samples were sieved and screened with sizes ranging 3 mm to 45 microns with the aid of vibratory screen. This was achieved with the aid of a mechanical sieve shaker/size analyser which comprises an agitator coupled with a mesh of various sizes, ranging from 3 mm to 45 microns. The samples were poured gently into the upper sieve while lowering the samples across the various mesh designations, to collect the finest samples at the receiver plate. The sieving and screening was done to prevent the abrasion of casing during its potential usage by coarser grain particles during drilling (Ibe et al. 2016).
XRD analysis (phase analysis) of the sample
The micronized samples of the crude and on-site processed barite minerals were analysed by the Bruker D8 Advance ECO A25 diffractometer with DAVINCI design. This was done on the reflection-transmission spinner stage using the Theta–Theta settings. The Two-Theta starting position starts from 15° and ends at 80° with a two-theta step of 0.0396 at 0.3 seconds per step. Energy requirement and details of the experimental procedure have been discussed extensively in the literature (Ayim and Enoch 2009; Olusegu et al. 2015). Files of d-spacing for hundreds of thousands of inorganic compounds are available from the International Centre for Diffraction Data as the Powder Diffraction File (PDF). Observable peaks obtained in the analyses were matched and analysed with ICDD database.
Determination of sample pH
A 20-gram sample of the sieved barite was placed in a beaker, and 20ml of distilled water was added to it. The slurry formed was allowed to stand for 70 minutes and stirred for about 10 minutes to measure the pH of the slurry (Ibe et al. 2016). The electrode remained immersed in the sample slurry until the meter stabilized. The pH was measured repeatedly after 24 h and specifically at the temperature range of 26.3 °C in line with the pH manufacturer’s instruction manual (Washington State Department of Transportation (WSDT) 2009; Ibe et al. 2016).
Determination of metals
The samples were digested in the mixture of chloroform (CHCl3) and 10% nitric acid (HNO3). The metallic content of the digested samples was analysed using atomic absorption spectrophotometer (AAS), Model: A-Analys 100. Liquid–liquid extraction method (LLEM) was employed in the absorption or digestion of the sample (Ibe et al. 2016).
Determination of sulphate
The method of IITA (1979) was adopted for the determination of the sulphate in the sample. This was done in line with the recommended specifications (Ibe et al. 2016; IITA 1979).
Cation exchange capacity and methylene blue absorption of Torkula barite
A 2 ml sample of the barite solution (barite fluid) was drawn into Erlenmeyer flask, and 10 ml of deionized water and 15 ml of 3% hydrogen peroxide (H2O2) were added to the drilling mud in the flask. Subsequently, 0.5 ml of 5N H2SO4 was added into the mixture, heated and allowed to boil gently for 10 minutes. The mixture was diluted with deionized water to 50 ml mark, and methylene blue solution was further added to the mixture in drops. Furthermore, the content was swirled manually for few seconds and while the solids are still suspended, the glass rod was inserted into the solution. The tip of the inserted glass rod was placed on the filter paper, and the drop of the mixture was carefully observed. The swirling, observation and addition of more drops of methylene blue solution was done continuously until a turquoise ring was seen on the filter paper (Burrafato and Miano 1993).
Determination of sample specific gravity
The specific gravity of the sample was determined in accordance with the American Standards Testing Methods ASTM D854-00 (ASTM 2015). Barite stones/aggregate were screened into different particle sizes. Test methods for specific gravity of soil solids by water pycnometer were used to calculate the specific gravity of the 75-µm barite samples (a fully compacted material) at water temperature of 25 °C. The specific gravity of unprocessed barite ores (aggregates) were also measured.
Determination of sample moisture content
Samples of 5, 10, and 15 g of the micronized barite were weighed into a dry and clean crucible, and placed in an oven, working at an operating temperature of 105 °C for 3 h. At the end of the heating period, the samples were removed from the oven and kept in a desiccator, to cool for about 3 h. The cooled samples were measured as the dried weight of the samples. The differential weights of the samples were compared and computed using an analytical weighing balance. The entire process was repeated after 3 h until a constant weight was obtained (Ibe et al. 2016).
Determination of sample Mohr’s hardness
Five (5) fully enriched lumps (un-pulverized) barite sample was scratched on the surface with the edge of a penny and an etched line was noticed. This was observed with the aid of a finger nail, to feel for a scratch and validate the nature of the scratch. If the barite is scratched, then it is softer than or equal in hardness to the test material, and if otherwise, it is harder than the test material. This method relies upon a scratch test in order to relate the hardness of a mineral specimen to a number from the Mohr’s scale (Alan 1997). The scale was defined using an assembled set of common reference minerals of varying hardness and labelled these in order of increasing hardness from 1 to 10.
Similarly, the samples may also be scratched against either a knife, Coin, glass or finger nail. The observed results were evaluated on the Mohr’s scale of minerals hardness (Ibe et al. 2016; Omoniyi and Mubarak 2014).
Determination of sample density (mud weight)
The required weight of the mud column establishes the density of the mud for any specific case. This is required as the starting point of pressure control of mud density and the balance of the formation pressure (Osokogwu et al. 2014). The mud balance comprises up a small vessel, filled with the mud and a metering section which was adjusted gently to account for the mud weight. This balance has two (2) different calibrations on it and was arranged in line with the recommended Specifications (Omoniyi and Mubarak 2014). The balance arm was positioned on the support base and was adjusted by moving the rider along the graduated scale until the level bubble was centred under the centre line. The density of the mud (mud weight) was measured and recorded appropriately. The cup outside was made cleaned and dry (Osokogwu et al. 2014).
Effect of the local barite on the filtration properties of the mud
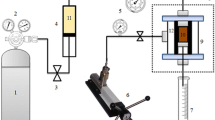
The presence of water in the filtrate indicates emulsion weakening, while thick filter cakes and high fluid loss indicate excessive drill solids content. The effect of the local barite on the mud filtration properties was investigated using a low-pressure low-temperature filter press. The lid was removed from the bottom of the clean and dry cell and the O-ring placed in an undamaged groove in order to seal the inlet with a finger and prevent any mechanical damaged. The cell was filled with the mud sample containing the local barite up to one-fourth (¼) of the O-ring groove, and a filter paper (Whatman No. 50 or equivalent) was placed on the top of the O-ring. The lid was placed on the filter paper with the flanges of the cell and turned clockwise until hand tight. A suitable graduated cylinder was placed under the filtrate opening to receive the filtrate. This was done for 30 minutes according to API test specification. The cell was disassembled, and the mud was discarded. The mud cake thickness was measured (Osokogwu et al. 2014).
Effect of barite on the rheological behaviour of the mud
The formulated drilling mud (based on Table 3) was measured into the thermal cup up to approximately 70% and positioned on viscometer stand. The cup was raised and stands until rotary sleeve was completely immersed to scribe line on sleeve locked into place by turning locking mechanism (Omoniyi and Mubarak 2014). Dial reading was delayed or otherwise paused for 20 s to stabilize. The process was repeated for 600, 300, 6 and 3 rpm, respectively. Finally, the dial readings were observed and recorded, respectively, in line with the recommended API specifications (Osokogwu et al. 2014).
Results and discussion
Phase analysis of Torkula barite
The X-ray diffractogram in Fig. 4 reveals the composition and crystallographic information of the crude and on-site processed barite. Identifiable peaks in the diffractogram were matched with d-values obtained from the ICPDS card number 00-024-0020, and JCPDS (Joint Committee on Powder Diffraction Standards) data of card number: 39-222. Similarly, the crystallographic open database (COD) card number identified indicated the most dominant form of occurrence for the barium (in different phases) and sulphate as most dominant elements. Other gangue minerals include quartz, calcite, and hematite. This is in line with reports given in the literature (Aladesanmi et al. 2018; Mgbemere et al. 2018a, b; Tanko et al. 2015).
Assessment of the chemical composition and properties of Torkula barite
Table 4 shows that the on-site processed Torkula barite has 87.79% BaSO4, 1.39% Fe2O3, 6.66% silicate salts, 1.603% total heavy metals, 0.589% CaO, 0.086% MgO, 0.75% Al2O3, and 1.10% LOI. The efficiency of the on-site beneficiation process adopted in Torkula barite field clearly justifies the upgrading of materials characteristics of the mined minerals and enhancement of the potency of the value addition strategies. Similarly, ICPMS and XRF results in Fig. 5 and Table 4 indicate the presence of silica and alumina based minerals (clay minerals), hematite and other heavy metals, as the most dominant impurities, while other gangue minerals in traces include iron, potassium, zinc, strontium, and magnesium. However, the percentage of impurities in the Torkula barite was low. Hence, quality of Torkula barite can be upgraded to compete with high grade barite for oil drilling and other industrial applications.
Alkalinity and hardness of the drilling mud as a function of Torkula barite
Alkalinity and hardness are traceable to the partitioning of CO2 from the atmosphere, weathering of rock, minerals, and soil. The phenolphthalein alkalinity of the drilling fluid formulated based on Torkula barite and other additives was at 1061 mg/L as shown in Table 5. This is the quantity of extractable carbonates in Torkula barite and is within the API specification of 3000 mg/kg. Hence, the carbonates in Torkula barite when combined with other compound may alter toxicity. On the other hand, the total alkalinity added up to 1658 mg/L, indicating the total amount of calcium hydroxide (lime) required to reduce the pH of the mud to 4.3 from the actual pH value of the drilling mud.
The carbonate alkalinity, chloride count, and mineral acidity of Torkula barite are relatively higher when compared with the American Petroleum Institute (API) Standards, as shown in Table 5. This may alter its toxicity and become detrimental to health except a scavenging element is introduced to reduce the health risk. Most importantly, high alkalinity of Torkula barite on the other hand may act to resist major change in the pH, and to control corrosion effectively.
Methylene blue absorption of Torkula barite mineral
Methylene blue absorption (MBA) studies measure the exact quantity of positively charged ion expressed in milliequivalents present in the drilling mud per 100 g of fully dehydrated clay mineral. Table 6 and Fig. 6 clearly examine the reactivity and absorption capacity of Torkula barite. The volume of methylene blue absorbed at different quantities of Torkula barite is 14 ml, representing clays base exchange capacity for the drilling fluid. This is because the quantity of aluminosilicate in 10 and 70 g of barite gave rise to the same methylene blue capacity. Hence, the MBA and cation exchange capacity (CEC) for 10 g and 70 g barite are the same (API 1995a, b).
Barite and other additives in the drilling mud contributed to 0.012 milliequivalent of reactive clays. This was due to low dissociation of the barite minerals. This may limit the performance of shale cuttings on the borehole stability, completion fluid properties, rheology enhancement and other mud properties. However, such observation might also result in the prevention of crevice corrosion, rendering Na+, K+, Ca2+, Mg2+ ion passive due to the inertness of the clay mineral. And so, the material properties of the barite sample are tailored to suit API specifications for Cl−, Fe2+, Fe3+(Brevier et al. 2002; Sterner 2002).
Mean metallic content of Torkula barite
The chemical, physical, and rheological responses of Torkula barite depend on the mean values of chemical parameters shown in Fig. 7a–g, respectively. These were compared with the American Petroleum Institute (API) and the Nigerian Department of Petroleum Resources (DPR) set limits. It is clearly shown in Table 7, Fig. 7a, b and d that lead (Pb) has the highest concentration in Torkula barite and is followed by calcium and iron minerals. It can thus be summarized that Torkula barite contains galena, calcite, hematite, pyrite, magnetite, sphalerite, brucite, chalcopyrite and chalcocite associated minerals with galena, calcite dominates the host of gangue minerals.
Figure 7b–g shows the exact value of the chemical parameters. The calcium (Ca), lead (Pb), zinc (Zn), magnesium (Mg), copper (Cu), and cadmium (Cd) minerals in Torkula barite were within the limits set by the American Petroleum Institute (API) and Nigerian Department of Petroleum Resources (DPR). Results of research work of Ibe et al. (2016) on the reference sample were compared with results obtained in this work. The quantity of iron mineral in Torkula barite was higher than the API minimum limits, however, lower when compared with the DPR acceptable limit (Ibe et al. 2016).
Hydrogen potentials of Torkula barite
The potential of hydrogen (pH) as a function of electromotive force (e.m.f.) was measured at a given temperature for a period of 24 h, as shown in Fig. 8f, g, respectively. Figure 8a clearly explains the rate of hydrogen dissociation of the unprocessed barite and the on-site processed sample in deionized water. The pH of barite in water was slightly alkaline after the initial 70 min, as shown in Fig. 8c. However, its alkalinity (hydroxyl ion, OH−) increased from 7.9 at 28.3 °C to 8.0 at 26.3 °C after 24 h.
a pH-Time graph for the unprocessed and processed Turkula barite sample from Guma LG A, Benue State, Nigeria, b–d comparison studies Torkula barite, industrially acceptable grade and barite deposits reported in the literature, e hydrogen dissociation stability of crude and processed Torkula barite (Us. SA-Un. SD: unprocessed barite samples A-D, On. SPS: on-the-site processed barite)
The analysed crude barite samples were slightly alkaline in water, and the status of their alkalinity increased from 8.286 to 8.643, 8.108 to 8.510, 8.156 to 8.507 and 8.153 to 8.474 for samples Un.SA, Un.SB, Un.SC, and Un.SD, respectively. The pH of barite samples is above the point of zero charge of pure barite. Thus, the barite particles could be easily dispersed in fluids to ensure slight increase in fluid salinity. At moderate fluid salinity, the quality of the filter cake is improved, the mud weight, fluid’s gel strength, and fluid loss to the formation become moderate. Conversely, the hydrogen dissociation of crude barite was clearly unstable. It increases as the hydrogen ion forms and decreases slightly when it is consumed during the chemical reaction. This clearly indicates the consumption of the hydrogen ion by activity of associated minerals present in the crude barite. Despite the shortfall in the pH of the sample, the measured values were within the alkaline range. This is shown in Fig. 8b, d. At these pH values, the solubility of calcium is very limited, and the drilling mud is suitable for use in the drilling of sandstone formations. This addresses the associated uncertainty and enhances the control measures to erosion and dissolution of freshwater mud. However, the active clays in the mud are easily disintegrated at high potential of hydrogen to modify materials properties of barite as weighting agent in the drilling mud (Azar and Samuel 2007; Christ 2006; Ibe et al. 2016).
The dissociation of the barite mineral after 24 h was minimal when compared with the reference sample and other Nigeria barite analysed by Ibe et al. (2016), Osokogwu et al. (2014), and Ádewale and Salihu (2014), as shown in Fig. 8d, e. This shows that the difference in the pH of Torkula barite after 24 h is not as significant, and as such is within the API recommendations of pH=7−≤12.5 (Christ 2006; Ibe et al. 2016; Osokogwu et al. 2014; Ádewale and Salihu 2014). Its application as a weighting agent in water drilling mud enhances the moderation of hydrogen potentials of the drilling mud within the acceptable limits stated by API. Hence, the pH of Torkula barite fell within the operational pH regime and standards range of drilling weighting agent.
According to Azar and Samuel (2007), and Christ (2006), at pH of < 9.5, the fatigue life of the drill stem is reduced drastically. Its alkalinity was enhanced by the addition of sodium hydroxide or lime and raised the pH to 10. This in turn is said to reduce the corrosion rate and diffusion of oxygen to the surface of metal, and to increase its performance in service (Azar and Samuel 2007; Christ 2006). The extent of alkalinity was slightly reduced from 8.6 in unprocessed barite sample A to 8.0 as observed with the on-site processed barite.
Figure 8c correctly reveals the pH stability potentials of Torkula barite. The differential hydrogen dissociation of the unprocessed samples was within 0.32 and 0.40 mol/dm3. However, this reaction products or constants were considerably reduced from 0.40 to 0.079 mol/dm3. At high hydrogen dissociation, the stability of the mud pH became unpredictable. Hence, the on-site beneficiation processes employed has reduced the hydrogen dissociation to enhance the pH stability of the drilling mud.
Physical properties of Torkula barite
Moisture content of Torkula barite
As shown in Fig. 9, the differential loss in weight of the crude and on-site processed barite was 0.076 and 0.0972, 0.1225 and 0.1610%, respectively. The average weight of the barite samples decreased from 0.1418 to 0.0866%. Similarly, the moisture content of barite samples doubled after processing. Hence, in comparison with the API standards, the values of moisture content of the barite samples under analysis are below the API maximum value of 1%. However, the moisture of the on-site processed barite is higher than the crude barite. This increase in the moisture content will affect the mud viscosity negatively and may result in the collapse of the borehole due to varying downhole conditions. Similar experimental observations were documented in the work done by Dhiman (2012), Ibrahim et al. (2017) and Osokogwu et al. (2014).
Estimation of the weight loss of Torkula barite samples (Un.SA, Un.SB: unprocessed barite ores, On.SPSA and On.SPSB: on-the-site processed barite samples, A–E on-the-site- processed barite samples after 3 years of storage, API Max: maximum acceptable percentage moisture content by American Petroleum Institute API)
Further experiment was done to examine the effect of long-time storage on the moisture content of barite samples. Figure 8 shows that the moisture content of barite samples has increased. Samples B, C, and D have their moisture content below 0.2%, which is very close to the moisture content 3 years ago. However, the moisture content of samples A and D is 0.36 and 0.7%. Thus, the moisture content of the samples is within the API standards and the long-term storage has no significant effect on the quality of the on-site processed barites
Specific gravity of barite samples
The distinctive specific gravity for each barite sample is shown in Fig. 10a, b. Solid samples C, D, and F have specific gravities of 3.94, 4.02, and 4.02, respectively. These values are less than the acceptable standards 4.10 SG, specified by the American Petroleum Institute. Samples A and B have specific gravity that are reasonably higher than the globally accepted standards of 4.10 SG but lower than the API 4.21 SG. All the samples have specific gravities that is good for normal drilling operation. These samples have a relatively low quantity of gangue minerals, as revealed in the chemical composition (Figs. 4, 5; Table 4) and can be further processed to the specific gravity to 4.21. Thus, these barite rocks or aggregate will add weight to the drilling fluid to ensure a balance of formation pressure during drilling operations.
a Comparison bar chart for the specific gravities of crude barite grade, locally processed barite grade, and other barite deposits/grade reported in the literature (Samples A–E: on-the-site processed barite samples; Unprocessed barite ore; Ibe et al. (2016): sample analysed and reported in the literature). b Comparison bar chart for the specific gravities of industrially accepted barite (Nigerian Department of Petroleum Resources), global drilling standards (API), and locally processed barite grades (NDPR Value: specific gravity of barite used by oil industries in Nigeria; API 4.2 SG and API 4.10: API standards, Samples A–E: on-the-site processed barite samples)
For the drilling fluid formulation, barite rocks or aggregates are ground or reduced to fine particles (3–74 µm). During the comminution process, aggregates are mixed and grinded as a bulk material. The milled samples have specific gravities above or below the require value due to the density of the non-barite minerals present in the ore. In this study, the specific gravity of the unprocessed barite is 4.02 ± 0.07. The on-site beneficiation process successfully upgraded the specific gravity of milled samples from 4.02 ± 0.07 to 4.15 ± 0.13. The specific gravities of unprocessed or crude barite, locally processed barite, and other barite deposits reported in the literature were compared, as shown in Fig. 10a. Similarly, the specific gravity of each locally processed barite was compared to the industrially accepted and global drilling standards for barite as a weighting material. Depending on the local processing methods, certain low-density minerals (impurities in the barite ore) can be carried away as tailings or trapped in the valuable minerals and reduce the specific gravity of the processed barite. Such low-density minerals were identified as shown in Figs. 4 and 7, and Table 4. These contaminants in the local barite can create drilling problems. Consequently, the mud system should be examined and evaluated to ensure that quality assurance and safety protocols are duly observed for drilling mud additives.
Hardness of barite samples
The hardness of six barite samples was measured using the scratch method. Samples A, C, and D have a mean value of Mohr’s hardness between 3.5 and 4, as shown in Fig. 11. Also, samples C and D have higher specific gravities and are close to the API SG standard. However, hardness values for samples B, E, and F were within the range of 4.5 and 5 Mohr’ hardness scale. Differences in the hardness Mohr scale are traceable to the nature of the associated or gangue minerals, as shown by different colours and texture. Weighting agents such as Torkula barite, having the Mohr’s hardness between 3 and 4, can sufficiently protect the drilling bit and preserve the circulation system. Beneficiation of samples A, B, E, and F to liberate or extract the gangue minerals from the host will enhance their rheological properties and hardness. However, when such is not addressed prior to drilling or cementing operation, the wear rate of the bore and the wear debris will be massive.
Correlation between the SG and Mohr’s hardness of Torkula barite
The qualities of Torkula barite were evaluated as shown in Figs. 10a, b and Fig. 11 on the basis of their specific gravity and Mohr’s hardness. These were validated through thorough comparison with API standards, the reference barite sample commonly used in most Nigeria’s indigenous oil and gas industries and other research works. Four out of seven barite samples from Torkula barite field in Benue State have SG greater than the API standard. These can be classified as high-grade barite, while two from the remaining three were within the medium grade. However, sample 2 (coloured barite) belongs to the lower grade class.
Similarly, the wear rate of the bore in drilling operations is largely traceable to the hardness of the barite mineral. Samples A, C, and D were soft, while B, E, and F were hard barite minerals. Barite samples B and C were softer than the industrially accepted barite (reference sample), and the two barite samples analysed by Ibe et al. (2016). These were classified based on the API standards. Conversely, this can be sufficiently corrected through the adoption of a well-designed washing bay and efficient separation mechanism.
Mud weight control parameter for Torkula barite
Mud weight is the exact mass per unit volume of an API drilling mud. It is measured to determine the mud column’s hydrostatic pressure required to enhance or control the pore and formation pressures. The significance of the specific gravity of the Torkula barite is clearly defined in Fig. 12c, e. Addition of the barite increased the weight of the drilling fluid. Increasing the quantity of Torkula barite from 5 to 100 g reflects an increase in the mud weight from 8.49 to 10.5 ppg. Figure 12c indicates that industrially acceptable barite increases mud weight from 8.3 to 8.4 ppg. In contrast, the barite under study increased the mud weight from 8.5 to 8.65 ppg which is up to API standard when barite addition was increased from 5 to 10 g. Based on the same drilling fluid formulation, the mud weights of fluids 1, 2, and 3 are higher than the values reported by Osokogwu et al. (2014), as shown in Table 8 (Osokogwu et al. 2014). The drilling fluids’ density formulated based on the use of Torkula barite has a higher mud weight. Thus, the mud weight is within the acceptable limit and cannot fracture a formation or slow the drilling bits’ intermediate rate.
Mud weight and filtration properties of the drilling fluids; (a rate of fluid/filtrate loss in 30 min, b linear correlation of the filtrate loss to obtain the API filtrate loss expression, c mud weight increase using Torkula barite and industrial accepted barite, d comparison of the filtrate/fluid loss reduction potentials of Torkula barite and industrial grade barite blended with fluid loss control materials (mica and aluminium) as reported in the literature, e significance of Torkula barite addition to the mud weight (formulation 2))
The Torkula Mud Weight test, as shown in Fig. 12e, resulted in the formulation of a mathematical expression to guild the industrial application of the weighting agent. Hence, such graphical analysis will serve as a fundamental control expression for drilling mud programme. Moreover, this study showed that Torkula barite could be blended with bentonite to increase the drilling mud weight efficiently. Torkula barite as a weighting agent in drilling fluid will sufficiently compete in functions, applications, and performance without resulting in an excess weight of the solids (Bourgoyne et al. 1986; Haaland and Pettersengier 1976; Joel 2013).
Quality of the filter cake as a function of API filter loss
The analysis on the quality of filter cake is shown in Fig. 12a, b and d. It was very obvious that the cumulative filtrate volume increases with time function. However, the volume of filtrate collected at the discrete time steps decreases gradually with time. This was justified with the quadratic nature of the filtration curve. Similarly, the volume of the filtrate lost increase with the square of time. The significance of the spurt loss is evident in Fig. 12a, b, respectively. The filtrate loss of 3ml was observed as the sporadic rise between 0 and 5 minutes and measured on the API scale to be 0.0056. The negative (minus sign) symbol in the API filter loss expression clearly describes the initial formation processes which gave rise to the filter cake. A thin, firm filter cake was obtained with 10g Torkula barite as shown in Table 9. Increasing the quantity of Torkula barite in the drilling mud reduces the spurt loss. However, the thickness of the filter cake was increased and became firm and flexible. The fluid loss to the formation of the filter cake increased, and the quality of the cake formed improved. These positive observations are traceable to the drilling fluid’s salinity based on the quantity of KCl used in the formulation. Hence, 10 g Torkula barite was used to enhance the filter cake quality and reduce the fluid loss. Locally processed barite performed better than the industrially acceptable barite, as shown in Fig. 12d. This is fundamental to the sealing of the formation pore by the restriction to influx and further invasion of the fluid (API RP 13B-1 1995b; Anyanwu and Mustapha 2016; Da Fonseca 2015; Anttila and Jussila 2017).
Effect of addition of Torkula barite on the rheology of the drilling fluid
Plastic viscosity
Plastic viscosity of the mud varies with the quantity of Torkula barite added as a weighting agent. It increased from 7 cP (0.007 Nsm−2) to 30 cP (0.030 Nsm−2) when the weight of Torkula barite was increased from 5 g to 10 g and reduced to 17.5 cP (0.0175 Nsm−2) as the weight increases from 10 to 70 g. Such observation revealed that plastic viscosity increases with the amount of solid and might also decrease when the solid content is in excess. The increase in plastic viscosity is initiated by a viscous base fluid and excess colloidal solids. The plastic viscosity of drilling fluid with 10 and 70 g of barite is above the API minimum value and within the range of values reported for the industrially acceptable drilling fluid as shown in Fig. 13 and Table 8. The low plastic viscosity of the drilling fluid with 70 g indicates that the mud is capable of being used in drilling operations due to the low viscosity of the mud exiting from the bit of the viscometer. Torkula barite can be used to increase the plastic viscosity of drilling mud to an acceptable limit. However, such drilling operations will be done at an intermediate rate of penetration (ROP).
Apparent viscosity
Apparent viscosity is measured based on the shear rate at a fixed temperature. The scale is shown as one-half of the dial reading at 600 RPM and specified by the American Petroleum Institute (API) Standards (API 2010). Its value depends on the shear rate and describes the amount of resistance to the flow when drilling fluid is sheared. The apparent viscosity of the drilling mud increases from 11 to 31.75 lb/100ft2 when the quantity of Torkula barite was increased by 5 g as shown in Table 8. The result indicates that the deformation rate of the fluid reduces and its ability to recover from such deformation is higher when the quantity of local barite in the fluid increases from 5 to 10 g. However, the plastic viscosity begins to experience a slight fall when an additional 60 g of local barite was added into the drilling mud. Figure 13 also shows that the apparent viscosity reduced from 31.75 to 29.5 lb/100ft2 as the barite quantity increased from 10 to 70 g. Such a situation will increase the solid content of the drilling mud. The higher the resistance to the flow when a fluid is sheared, the greater its ability to remove drill-cutting held due to high hydrostatic pressure and ensure drilling of new formation. Torkula barite is compatible with other additives in the drilling fluid, and the apparent viscosities of the fluids is sufficient to prevent various drilling problems, improve oil well cleaning efficiency, and suitable for specialized drilling operations within a control volume of liquid and solid content (Robert et al. 2012).
Yield point
This is the initial resistance to flow induced by electrochemical forces between the solid content particles constituting a drilling fluid. Such parameter is graphically obtained from the Bingham plastic fluid plot as the zero-shear-rate intercept on the shear stress axis (Abduo et al. 2015). Contrary to the drilling fluid’s response in terms of the plastic viscosity, the additional increase in Torkula barite from 5 to 10 g reflected a slight fall in the yield point of the drilling fluid. However, the increase in yield point from 3.5l to 24l b/100ft3 resulted from the additional 60 g barite that was added to the mud. This increase and decrease in the yield point indicate the level of the electrochemical forces in the drilling fluid and directly depends on the surface charges of particles in the fluid. As the local barite quantity increases from 5 to 10 g, the attractive forces were reduced by a specific chemical reaction between solid particles in the fluid. Figure 13 also indicates that the yield point of the drilling fluids (formulation 2) prepared with 5, 10, and 70 g of local barite were lower than the API minimum requirement. The drilling fluids’ ability to suspend and remove solids from the wellbore is low or poor and can be improved. The contribution of Torkula barite as a weighting agent depends on the surface properties, the dynamic conditions, and volume concentration of the solid components. Increasing the quantity of Torkula barite in a drilling mud will increase the attractive forces and the ability of such non-Newtonian fluid to lift cutting, and clean the wellbore.
Gel strength
This is the shear stress measured at a low shear rate, after which the drilling fluid has set quiescently and is within the period stipulated by API. Such fluid property demonstrates the ability to suspend drill solids during a halt in drilling operations. The emulsion gel structure formed by the drilling fluid composed of 5 g of barite was more stable and increased as the quantity of barite added into the drilling fluid increased. As the concentration of solids or barite particles in the fluid increased, the attractive forces or extent of the drilling fluid’s gelation increases under static conditions. However, high solid or barite concentration may result in flocs formation when the fluid pH is close to the point of zero charges of some solids in the fluid. In such a situation, a low-flat gel curve is produced. Table 8 shows that the gel strength of the drilling fluid increased from 2.5 to 3.5 lb/100ft2 as the barite quantity increases from 5 to 70 g. This implies that the addition of Torkula barite into the drilling mud increases gel strength slightly.
Tables 10, 11, and 12 indicate that the fluid’s viscosity increases as the shear rate decreases. The fluids can form gels in a static situation and flow when the fluid is sheared during flow. Also, the gel strength of fluids is relevant in assessing risk and design of safety protocols that guarantee safe drilling activities. The gel strength values obtained in this study are ideal for ordinary drilling operations but low for specialized operation. In a general, during normal drilling operation, the fluid has sufficient strength to ensure that the mud column’s pressure is maintained and kept below the differential pressure between the mud and formation fluids to prevent blowout. However, in specialized operation, the gel strength must be increased to enhance the suspension of cuttings, prevent pipe stuck and hole pack off, and barite sag. This can be done by increasing the quantity of barite dosage or adding a viscosifier to adjust the point of zero charges (PZC) of the fluid. Thus, Torkula barite can produce fluids with flat and thixotropic gels (Herzhaft et al. 2006; Marsh 1931; Abduo et al. 2015; Anyanwu and Mustapha 2016; Werner et al. 2017).
Addition of barite to the mud sample had impact on the shear stress and viscosity behaviour of the mud. Figure 14a, b shows the shear stress to shear rate relationship and viscosity to shear rate relationship of the mud samples with local barite as a weighting agent. It was observed that the viscosity of the mud decreases as a function of increasing shear rate. There was a sharp drop in viscosity as the shear rate is increased in the fluid with higher proportion of barite. Similarly, it is confirmed in Fig. 14a that the properties of the fluid changes from a pseudoplastic to plastic fluid as the amount of barite was increased from 5 to 10 g and then 70 g, respectively. This confirms that the three-dimensional complex structure breaks down due to the applied external force and results in shear thinning behaviour. However, as the quantity of barite is increased, the fluid exhibit yield value and demonstrate shear thinning with increasing shear rate behaviour. The sudden change in the structural characteristics of the fluid due to increased barite content suggest that the solids in the fluid strengthens the inter-particle or molecular association, but the structure might still be disrupted, and the position of the particles disturbed at a given shear stress.
Figures 14b and 15 clearly show the flow profiles of fluid 1, 2, and 3, respectively. In Fig. 14b, the viscosity is observed as a function of shear rate, while viscosity as a function of shear stress is plotted in Fig. 15. The fluids exhibit the same viscosity at a certain shear rate but behave differently when applied. Similarly, there was a sharp decrease in the viscosities of the fluids on the application of external force due to the breaking of the three-dimensional complex structure and results in shear thinning. Also, the viscosity of the fluids was higher at low shear rate and shear stress, while the viscosity of fluid 2 remains stable as the shear stress is increased beyond 180 mPa. There is an indication that fluid 2 can withstand higher external force, stain the stable three-dimensional complex structure beyond the application and reconstruction stages. However, fluids 1 and 3 may not be able to retain the complex structure beyond the formulation and production stages. Similarly, it was observed that the decreasing viscosity became steady as the quantity of solid weighting agent is increased from 5 to 70 g. This confirms a gradual transition from pseudoplastic fluid to plastic fluid as the quantity of solid weighting material is increased in the fluid formulation.
Contributions of Torkula barite to the fluid properties
Torkula barite water-based drilling mud (WBM) can be described efficiently in relation to its behavioural change at different dial readings. Figure 14 and Table 10 clearly reveal the response of the fluid at different mixing speed. The figure also shows an increase in the dial reading at 600 revolutions per minute (RPM) and a decrease in the dial reading at 300 RPM, when the quantity of barite is increased from 10 to 70 g. However, the dial readings at both mixing speed were higher than the minimum specification recommended by the American Petroleum Institute (API). The information obtained at different speed suggested that the drilling mud belongs to a class of non-Newtonian fluids. Such reflects a higher viscosity at low shear rate (RPM X 1.703) and a lower viscosity at high-shear rate. From the information in Fig. 14, the shear stress at each mixing speed, plastic viscosity (PV) and the yield point can be calculated. Hence, Torkula barite can be used to enhance the fluid properties required for both ordinary and specialized drilling operations.
The filtrate or fluid loss from the drilling mud prepared with Torkula barite is lower when compared with the API specification and other drilling mud formulated using the imported barite. However, it is within the acceptable standards. The low value obtained is a function of the amount and particle-size of solids (barite and bentonite) present in the mud. Hence, the field-scale beneficiation strategies employed on Torkula barite have greatly improved the quality of the mineral and its acceptance on the basis of the API regulations. The filtrate loss within thirty (30) minutes for Torkula barite is 7.7 ml as it is shown in Fig. 14, while that of the reference sample is 11 ml and the API Specification, which is at 12 ml. This attested that Torkula barite can sufficiently conserve the liquid content of the drilling mud due to its ability to form a good blend with other additives used in three different formulations (Abduo et al. 2015; Al-Bagoury and Steele 2012).
The calculated values that correctly described the measure of the internal resistance to the flow of formation fluid into the borehole during the drilling operations are clearly presented in Fig. 14. This shows the relationship between the fluid flow parameters. The apparent and plastic viscosities of the drilling fluid consisting of Torkula barite are higher than the API minimum values. However, these values were observed to be at the extreme end, and as such may be considered most appropriate even when there is an unexpected dilution of the fluid from a water ways. Similarly, the yield point of the fluid composed of 10 and 70 g barite is lower when compared with the API Specification and the value obtained with the imported barite. Furthermore, the yield point observed on the addition of 10 g barite is too low, while an average value was obtained when the amount of the weighting agent (Torkula barite) was increased from 10 to 70 g. Hence, higher amount of Torkula barite is required to raise the yield point of the drilling mud. On the other hand, these velocities were measured at different shear rates to determine the rheology model coefficients. They indicated the shear stress as a dial unit or degree at a given shear rate (API 2010; AMSE 2005).
Conclusions
The pH of the Torkula barite is within the operational pH and the standard range for a weighting agent, as recommended by the American Petroleum Institute. It is appropriate for long-time storage without any operational adverse effect on the drilling mud. Similarly, the moisture content of 0.118% is within the API standards and specification of 1% at maximum. In addition, the differential loss in weight of the crude and on-site processed barite was at 0.076% and 0.0972, 0.1225 and 0.1610%, respectively. All of these material’s properties clearly describe the nature and the structure of the barite–water interface, and any observable changes in such material’s properties affect the mud viscosity. This is also responsible for the increase in alkalinity and unstable hydrogen dissociation potentials of barite in the drilling mud.
The on-site beneficiation process upgraded the specific gravity of milled samples from 3.82 to 4.13. Barite sample 2 (coloured barite) and unprocessed barite sample A have a specific gravity of 3.85 due to the presence of undesired minerals. These associated minerals are galena, calcite, hematite, pyrite, magnetite, sphalerite, brucite, chalcopyrite, and chalcocite. Six among the seven samples had a little higher SG when compared to the samples analysed by Ibe et al. 2016. These are below the API standard of 4.2 SG. On-site processed barite has low iron, copper, cadmium, and lead content compared to the imported barite.
The carbonate alkalinity and barite mineral acidity were relatively high when compared with the American Petroleum Institute (API) Standards. This may result in some health risk risks and environmental hazards. Better still, the alkaline range of Torkula barite in water can be an advantage in the prevention of corrosion. However, the effluent from such processes must be treated and ensure that the level of toxic heavy metals such as Zn, Cu, Ba, Cd, Pb, and Fe is within limits recommended by WHO, USEPA, EU and Nigerian Industrial Standard (NIS).
The metallic content such as Ca, Pb, Zn, Mg, Cu, and Cd minerals, and extractable carbonates in Torkula barite was within the limits set by the American Petroleum Institute (API) and Nigerian Department of Petroleum Resources (DPR). However, the quantity of iron mineral in Torkula barite is higher than the API set limits but low when compared with the DPR set limit. Appropriate tailoring of these properties to suit national and global standards will qualify the use of Torkula barite mineral in ordinary and specialized drilling operations.
The rheological properties of drilling fluids formulated based on the local barite have been fully described. The plastic and apparent viscosity, yield point, gel strength, mud weight of the fluid, filtration, and thixotropic properties of the fluid are within the API limits. Addition of local barite into the drilling mud improves the rheology and thixotropic properties. This study has identified and expatiated certain relevant information the drilling fluid engineers and other end users need to know in their bid to explore the potentials of the locally processed barite. Useful recommendations and suggestions are provided in the paper on the necessary adjustments or additional materials required to improve the performance of the weighting agent and mitigate operational impacts in an normal and specialized drilling operation.
References
Abduo MI, Dahab AS, Abuseda H, AbdulAziz AM, Elhossieny MS (2015) Comparative study of using water-based mud containing multiwall carbon nanotubes versus oil-based mud in HPHT fields. Elsevier Publisher, Egyptian Petroleum Research Institute, Egyptian Journal of Petroleum, pp. 1–4. https://doi.org/10.1016/j.ejpe.2015.10.008
Achusim-Udenko AC, Gerald O, Martins O, Ausaji A (2011) Flotation recovery of barite from ore using palm bunch based collector. Int J Chem Sci 9:1518–1524
Ádewale OO, Salihu M (2014) Potential usage of local weighting materials in drilling fluid a substitute to barite. Int J Innovative Res Dev 3(13), ISSN 2278 – 0211 (Online), pp 1
Afolayan DO (2017) Field work at PIN mining company, field report, pp 1–5 (Unpublished)
Al-Bagoury M, Steele C (2012) A new alternative weight material for drilling fluids. IADC/SPE drilling conference and exhibition, 6–8 March, 2012, San Diego, California, USA
Aladesanmi AO, Ogundana AK, Olowookere AA, Jenakumo L (2018) Geological characterization of Azara barite mineralization, Middle Benue Trough Nigeria. J Environ Earth Sci 8(3):44–46
Alan E (1997) Mineralogy of meteorite groups. Meteorit Planet Sci 32(2):231–247
AMSE Shale Shaker committee (2005) The drilling fluids processing Handbook
Anyanwu C, Mustapha M (2016) Experimental evaluation of particle sizing in drilling fluid to minimize filtrate losses and formation damage. Society of Petroleum Engineers, SPE-184303-MS, SPE copyright, p 10
American Petroleum Institute (1995a) Recommended practice standard procedure for laboratory testing of drilling fluids, 5th ed. Washington, DC, USA
American Petroleum Institute (1995b) Schlumberger Oilfield Glossary, “, API RP 131, (2004), Methylene Blue Test for Drill Solids and Commercial Bentonites”, Section 12 in: API Recommended Practices 131: Laboratory Testing of Drilling Fluids, 7th ed, and ISO 10416:2002; American Petroleum Institute, pp 34–38
Anttila J, Jussila K (2017) ISO 9001: 2015–a questionable reform. What should the implementing organisations understand and do? Total Qual Manag Bus Excel 28(9–10):1090–1105
API (2004) Specification for drilling–fluid materials, API specification 13A, 16th edn, February, 2004, pp 13, 165
API (2010) Specification for drilling–fluid materials, API specification 13A, 18th edn, August, 2010, pp 5–27
ASTM (2015) standards test method for specific gravity of soil solids by gas pycnometer, ASTM Committee D18 on Soil and Rock, texture, plasticity and density characteristics of soils, D5550-14, pp 1–3. https://doi.org/10.1520/D5550-14
Ayim FM, Enoch E (2009) An appraisal of locally processed barite for use as weighting material for oil and gas well drilling in Nigeria. PTDF J Int J 2:1–3 (ISSN 1595–9104)
Azar JJ, Samuel R (2007) Drilling engineering, Tulsa, Oklahoma: Penn Well Corporation, Committee, S. S. S. (2001), Drilling fluids processing handbook, S. I.: Elsevier, pp 31–17
Bourgoyne AT, Millheim KK, Chenevert ME, Young FS (1986) Applied drilling engineering (vol 2, p 514). Richardson, TX: Society of Petroleum Engineers, vol 2, pp 51, 66–68
Brevier J, Herzaft B, Mueller N (2002) Gas chromatography—mass spectrometry (GCMS)—a new wellsite tool for continuous C1-C8 gas measurement in drilling mud—including first original gas extractor and gas line concepts, first results and potential. In: Paper J presented at the 2002 SPWLA annual logging symposium, Oiso, Japan, 2–5 June
Burrafato G, Miano F (1993) Determination of the cation exchange capacity of clays by surface tension measurements. Clay Miner 28:475
Chaanda MS, Obaje NG, Moumouni A, Goki NG, Law UA (2010) Environmental impact of artisanal mining of Barytes in Azara Area, Middle Benue Trough Nigeria. J Earth Sci 4(1):38–42
Chen C, Ren Z, Gao H, Zheng R, Jin Y, Niu C (2019a) Flotation studies of fluorite and barite with sodium sulfonate and sodium hexametaphosphate. J Mater Res Technol 8(1):1267–1273
Chen X, Gu G, Liu D, Zhu R (2019b) The flotation separation of barite calcite using sodium silicate depressant in the presence of sodium dodecylsulphate. Physicochem Probl Miner Process 55(2):346–355
Christ NI (2006) The Treatment of Drill Cutting Using Dispersion by Chemical Reaction, Technologies Partners International (Nig.) Limited publisher, 4p
Da Fonseca LMCM (2015) ISO 14001: 2015: An improved tool for sustainability. J Ind Eng Manag 8(1):37–50
Deng J, Liu C, Yang S, Li H, Liu Y (2019) Flotation separation of barite from calcite using acidified water glass as the depressant. Colloids Surf A 579:2019
Dhiman AS (2012) Rheological properties and corrosion characteristics of drilling mud additives. MSc thesis, pp 17 (Ken AP, Clifford JH (2001) Circular No. 4, Mineral Resource Engineer, pp 2, 3)
Essalhi M, Mrani D, Essalhi A, Toummite A, Ali-Ammar H (2018) Evidence of a high quality barite in Drâa-Tafilalet region, Morocco: a nonupgraded potential. J Mater Environ Sci 9:1366–1378
Fatoye FB, Ibitomi MA, Omada JI (2014) Barytes mineralization in Nigeria: occurrences and economic prospective, international. J Adv Sci Tech Res 1(4): 484. http://www.rspublication.com/ijst/index.html. ISSN 2249-9954
Garside M (2020) Global barite production from 2011 to 2019. https://www.statista.com/statistics/799487/global-barite-production/
Grigorova I, Dzhamyarov S, Nishkov I (2015) Barite floatation concentrate from Kremikovtzi “Black” Tailings. J Int Sci Publ 9:561, 563. ISSN 1314-7269
Haaland E, Pettersengier T (1976) “Testing of iHaaland, Ellen, Pettersengier, & Tuntland (1976), “Testing of iHerzhaft, B., Ragouillaux, A.and Coussot, P. (2006), "How to Unify Low-Shear-Rate Rheology and Gel Properties of Drilling Muds," IADC/SPE Drilling Conference, Miami, Florida, SPE 99080,
Herzhaft B, Ragouillaux A, Coussot P (2006) How to unify low-shear-rate rheology and gel properties of drilling muds. In: IADC/SPE drilling conference, Miami, Florida, SPE 99080
Ibe KA, Ogeleka DF, Ani IC, Uyebi GO (2016) Suitability of Nigerian barite as a weighting agent in drilling mud. Int J Mining Min Eng 7(1):51
Ibrahim DS, Amer NS, Balasubramanian N (2017) Effect of barite and gas oil drilling fluid additives on the reservoir rock characteristics. J Pet Exp Prod Technol 7(1):281–292
IITA (1979), Selected methods for soil and plant analysis. Manual Series 1, IITA Press, Ibadan, pp10–50
Joel OF (2013) Tapping the untapped wealth in our backyard: Pathway to local content development. Inaugural Lecture, University of Port Harcourt, River State, pp 7–9
Labe NA, Ogunleye PO, Ibrahim AA, Fajulugbe T, Gbadema ST (2018) Review of the occurrence and structural controls of Barite resources of Nigeria. J Degrad Min Lands Manag 3:1207–1216. https://doi.org/10.15243/jdmlm.2018.053.1207
Liu C, Song S, Li H (2019) Selective flotation of fluorite from barite using trisodium phosphate as a depressant. Miner Eng 134:390–393
Marsh HN (1931) Properties and Treatment of Rotary Mud. Trans AIME 92 (01):234–251
Mgbemere HE, Obidiegwu EO, Obareki E (2018) Beneficiation of Azara barite using a combination of jigging, froth flotation and leaching. Niger J Technol 37(4):957–962
Mgbemere HE, Hassan SB, Sunmola JA (2018) Beneficiation of Barite ore from Azara Nasarawa State, Nigeria, Using Froth Flotation. Niger J Technol Dev 16(1):43–47
MMSD (2010) Ministry of mines and steel development: barytes exploration opportunities in Nigeria, September mineral handbook, printed at MMSD, Abuja, Nigeria, pp 3–10
MMSD (2016) Investment Promotion Brochure August 2016, Ministry of Mines and Steel Development. Retrieved from http://www.minesandsteel.gov.ng/wpcontent/uploads/2016/09/Nigeria-Ministry-of-Solid-MineralsInvestment-BrochureV11.pdf
MMSD (2020) Ministry of mines and steel development: federal government to save $300 million from barite importation, February and July bulletin, MMSD, Abuja, Nigeria, pp 1–5
Nigeria Geological Survey Agency (NGSA) (2010) Baryte: exploration opportunities in Nigeria, Revised edition. Printed by Ministry of Mines and Steel Development, Abuja, pp 1–14
Nzeh NS, Hassan SB (2017) Gravity separation and leaching beneficiation study on Azara Nasarawa barite mineral ore. Glob J Res Eng J Gen Eng 17(5):41–45
Oden MI (2012) Barite veins in the benue trough: field characteristics, the quality issue and some tectonic implications. Environ Nat Resour Res 2(2):26
Olusegu F, Yaya I, Jude E (2015) Characterizing Barite from Bukkuyum local government area of Zamfara state of Nigeria, using Empyrean diffractometer DY 674 (2010) for XRD phase analysis of the powdered sample. World Acad Res J 1(2):6–9 (ISSN 5042-7318)
Omoniyi OÁ, Mubarak S (2014) Potential usage of local weighting materials in drilling fluid a substitute to barite. Int J Innov Res 3(13):493 (ISSN 2278-0211)
Onwualu AP, Inyang AE, Olife IC, Obassi E (eds) (2013) Unlocking the potentials of Nigeria's non-oil sector. Raw Materials Research and Development Council, Nigeria
Osokogwu U, Ajienka JA, Okon AN (2014) Evaluating the Effects of Additives on Drilling Fluid Characteristics www.ijesrt.com(C)International. J Eng Sci Res Technol 3(6):676–687 (ISSN: 2277-9655)
Raju GB, Ratchambigai S, Rao MA, Vasumathi N, Vijaya Kumar TV, Prabhakar S, Rao SS (2016) Beneficiation of barite dumps by flotation column; lab-scale studies to commercial production. Trans Indian Inst Met 69(1):75–81
Researchdrive (2020) Market barite report, pp 1–10. https://www.researchdive.com/38/barite-market
Robert F, Alan M, Philip P (2012) Fluid mechanics, 8th edn. Wiley, pp 76–83. ISBN 978-1-118-02641-0
Sombo T, Ayaakaa DT, Utah EU (2016) Assessment of Radioactivity and Health Implications of Some Surface Soils in Guma Local Government Area of Benue State, North Central. Nigeria. Asian J Eng Technol 4(5):2 (ISSN: 2321-2462)
Sterner M (2002) Internal marketing literature. Fluid Inclusion Technology Inc., Tulsa
Tanko IY, Adam M, Shettima B (2015) Petrology and geochemistry of barite mineralisation around Azara, North Central Nigeria. Int J Sci Tech Res 4(5):1. ISSN 2277-8616 44. IJSTR©2015 www.ijstr.org
U.S. Geological Survey (USGS) (2017) Critical mineral resources of the United States, Barite: mineral commodity summaries, professional paper 1802, U.S. Geological Survey, Reston, VA, pp 30–31. ISBM: 978-1-4113-3991-0. https://doi.org/10.3133/pp1802A
U.S. Geological Survey (USGS) (2019) Barite: mineral commodity summaries, prepared by Michele E. McRae, U.S. Geological Survey, VA, pp 28–19
U.S. Geological Survey (2020) Mineral commodity summaries 2020: U.S. Geological Survey, 200p. https://doi.org/10.3133/mcs2020
Wang HJ, Dai HX, Yang WL, Li TT (2014) Research on the flotation experiment of a low-grade Barite Ore in Myanmar. Appl Mech Mater 644–650:5277–5280
Washington State Department of Transportation (WSDT) (2009) WSDOT test method T 417 method of test for determining minimum resistivity and pH of soil and water, Engineering and Regional Operations State Materials Laboratory, DC, pp 1–8
Werner B, Myrseth V, Saasen A (2017) Viscoelastic properties of drilling fluids and their influence on cuttings transport. J Pet Sci Eng S0920-4105(16)31041-5. https://doi.org/10.1016/j.petrol.2017.06.063, PETROL 4075
Zhao Y, Liu S, Li X, Li T, Hou K (2014) Recovery of low-grade barite ore by flotation in the Southwest area of China. Appl Mech Mater 543–547:3865–3868
Acknowledgements
This study was funded by Regional Scholarship Innovation Fund (RSIF) and supported by Pan African Materials Institute (PAMI), Worcester Polytechnic Institute (WPI), and African University of Science and Technology (AUST).
Funding
Authors are grateful to the Regional Scholarship Innovation Fund (RSIF-PASET) for the offer of Ph.D. scholarship to the first author. Authors also appreciate the support of AfDB drilling laboratory, AUST, Nigeria, Pan African Material Institute (PAMI), and the Environmental laboratory, Kaven Hall, WPI, Worcester, MA, USA, in providing us an enabling environment for the laboratory work.
Author information
Authors and Affiliations
Contributions
David Oluwasegun Afolayan, Adelana Rasaq Adetunji, and Richard Kwasi Amankwah contributed to conceptualization. : David Oluwasegun Afolayan and Adelana Rasaq Adetunji contributed to methodology; David Oluwasegun Afolayan and Ogenerhumen Ogolo were involved in the formal analysis and investigation; David Oluwasegun Afolayan was involved in the writing—original draft preparation; David Oluwasegun Afolayan, Adelana Rasaq Adetunji, Ogenerhumen Ogolo, Peter Azikiwe Onwualu, and Richard Kwasi Amankwah were involved in the writing—review and editing; David Oluwasegun Afolayan contributed to the funding acquisition; David Oluwasegun Afolayan contributed to the resources; Richard Kwasi Amankwah, Adelana Rasaq Adetunji, and Peter Azikiwe Onwualu were involved in the supervision.
Corresponding author
Ethics declarations
Conflict of interest
The author declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Afolayan, D.O., Adetunji, A.R., Onwualu, A.P. et al. Characterization of barite reserves in Nigeria for use as weighting agent in drilling fluid. J Petrol Explor Prod Technol 11, 2157–2178 (2021). https://doi.org/10.1007/s13202-021-01164-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-021-01164-8