Assessing the Extinction Probability of the Purple-winged Ground Dove, an Enigmatic Bamboo Specialist
- 1Ecology and Environment Research Centre (EERC), Department of Natural Sciences, Manchester Metropolitan University, Manchester, United Kingdom
- 2Cornell Lab of Ornithology, Cornell University, Ithaca, NY, United States
- 3Laboratorio de Ecología, Comportamiento y Sonidos Naturales, Instituto de Bio y Geociencias del Noroeste Argentino (IBIGEO-CONICET), Salta, Argentina
- 4Programa de Pós-Graduação em Ecologia e Monitoramento Ambiental, Centro de Ciências Aplicadas e Educação, Universidade Federal da Paraíba, Rio Tinto, Brazil
- 5Programa de Pós-graduação em Ciências Biológicas, Universidade Estadual de Londrina, Londrina, Brazil
- 6Independent Researcher, Rio de Janeiro, Brazil
- 7Centre for Conservation of Atlantic Forest Birds, Parque das Aves1, Foz do Iguaçu, Brazil
- 8Seção de Aves, Museu de Zoologia da Universidade de São Paulo, São Paulo, Brazil
The continued loss, fragmentation, and degradation of forest habitats are driving an extinction crisis for tropical and subtropical bird species. This loss is particularly acute in the Atlantic Forest of South America, where it is unclear whether several endemic bird species are extinct or extant. We collate and model spatiotemporal distributional data for one such “lost” species, the Purple-winged Ground Dove Paraclaravis geoffroyi, a Critically Endangered endemic of the Atlantic Forest biome, which is nomadic and apparently dependent on masting bamboo stands. We compared its patterns of occurrence with that of a rare “control” forest pigeon, the Violaceous Quail-Dove Geotrygon violacea, which occurs in regional sympatry. We also solicit information from aviculturists who formerly kept the species. We find that the two species share a similar historical recording rate but can find no documentary evidence (i.e., specimens, photos, video, sound recordings) for the persistence of Purple-winged Ground Dove in the wild after the 1980s, despite periodic sighting records, and after which time citizen scientists frequently documented the control species in the wild. Assessments of the probability that the species is extant are sensitive to the method of analysis, and whether records lacking documentary evidence are considered credible. Analysis of the temporal sequence of past records reveals the extent of the historical range contraction of the Purple-winged Ground Dove, while our species distribution model highlights the geographic search priorities for field ornithologists hoping to rediscover the species—aided by the first recording of the species vocalizations which we obtained from interviews with aviculturists. Our interviews also revealed that the species persisted in captivity from the 1970s until the 1990s (up to 150 birds), until a law was passed obstructing captive breeding efforts by private individuals, putting an end to perhaps the best chance we had to save the species from extinction.
Introduction
Understanding the magnitude of global biodiversity loss is a fundamental goal for conservation, but ascertaining whether a species is extinct or extant becomes extremely problematic the rarer a species becomes (Diamond, 1987). Rediscoveries of “missing” species happen not infrequently; for example, at a rate of approximately 30% of mammals “claimed or suspected to be extinct” (Fisher, 2011; Fisher and Blomberg, 2011). Local and Global Red Lists reporting extinctions must balance this uncertainty, given that failure to report an extinction leads to conservative estimates of the current extinction crisis and false reporting of extinction may lead to the withdrawal of conservation support for a still-extant Critically Endangered (CR) taxon (Collar, 1998). Red Lists tend to be conservative, so missing species are in many cases not formally listed as extinct for decades after the last sighting. The problem of ascertaining species persistence is most acute in the humid tropics where species richness and extinction rates are highest and where field surveys have traditionally been least intense (Butchart et al., 2018). Extensive targeted field surveys are typically the best way of rediscovering “lost” taxa yet these surveys are often expensive and difficult to implement for low-density wide-ranging species. Thus, in many cases, it may be important to find methodologies to make better use of existing occurrence data (Newbold, 2010).
Birds are the best known of the world’s biota but even in this Class, species discoveries and rediscoveries are an annual occurrence (Scheffers et al., 2011). The Atlantic Forest of eastern South America has been the concurrent scene of both the suspected global extinction of a number of threatened species and the discovery of entirely new bird species to science (Lees and Pimm, 2015). Most of the species for which there are few or no confirmed recent records are microendemics. One new species, the Cryptic Treehunter (Cichlocolaptes mazarbarnetti) was formally described after its suspected global extinction (Mazar Barnett and Buzzetti, 2014; Butchart et al., 2018). One forest-associated species, the Purple-winged Ground Dove (Paraclaravis geoffroyi), stands out as anomalous given that it has a broad geographic range size spanning 644,000 km2 in the Atlantic Forest of Brazil, Argentina, and Paraguay (Figure 1; BirdLife International, 2021). This species is thought to be nomadic, in Argentina following flowering events of only two species of bamboos: takuarusu (Guadua chacoensis) and yatevo (G. trinii; Areta et al., 2009). The species was historically found with some regularity within its wide range, with Göeldi (1894) reporting the species to be common in the lowlands around Guanabara Bay in Rio de Janeiro. It is now listed as Critically Endangered on the IUCN Red List (BirdLife International, 2021) and Critically Endangered (Possibly Extinct) on the Brazilian Red List (MMA, 2014) following a long decline thought to be driven by the loss, fragmentation, and degradation of its forest habitats, exacerbated by its specialized nomadic lifestyle (Areta et al., 2009; Areta and Cockle, 2012). Collar et al. (1992) considered it to be “now close to extinction,” although it continues to be reported periodically and Butchart et al. (2018) calculated a probability that the species is extant of 0.9 (where 1.0 is definitely extant). Its probability of extinction in the next 100 years was calculated via a different method as 0.8 (Andermann et al., 2021). Purple-winged Ground Doves occur in sympatry with the superficially similar Blue Ground Dove (Claravis pretiosa) and the two species can be easily confused with inadequate field views, which may lead to false-positive or false-negative detections of the rarer species. The lack of sound recordings of Purple-winged Ground Doves constitutes a main obstacle for active and passive searches.
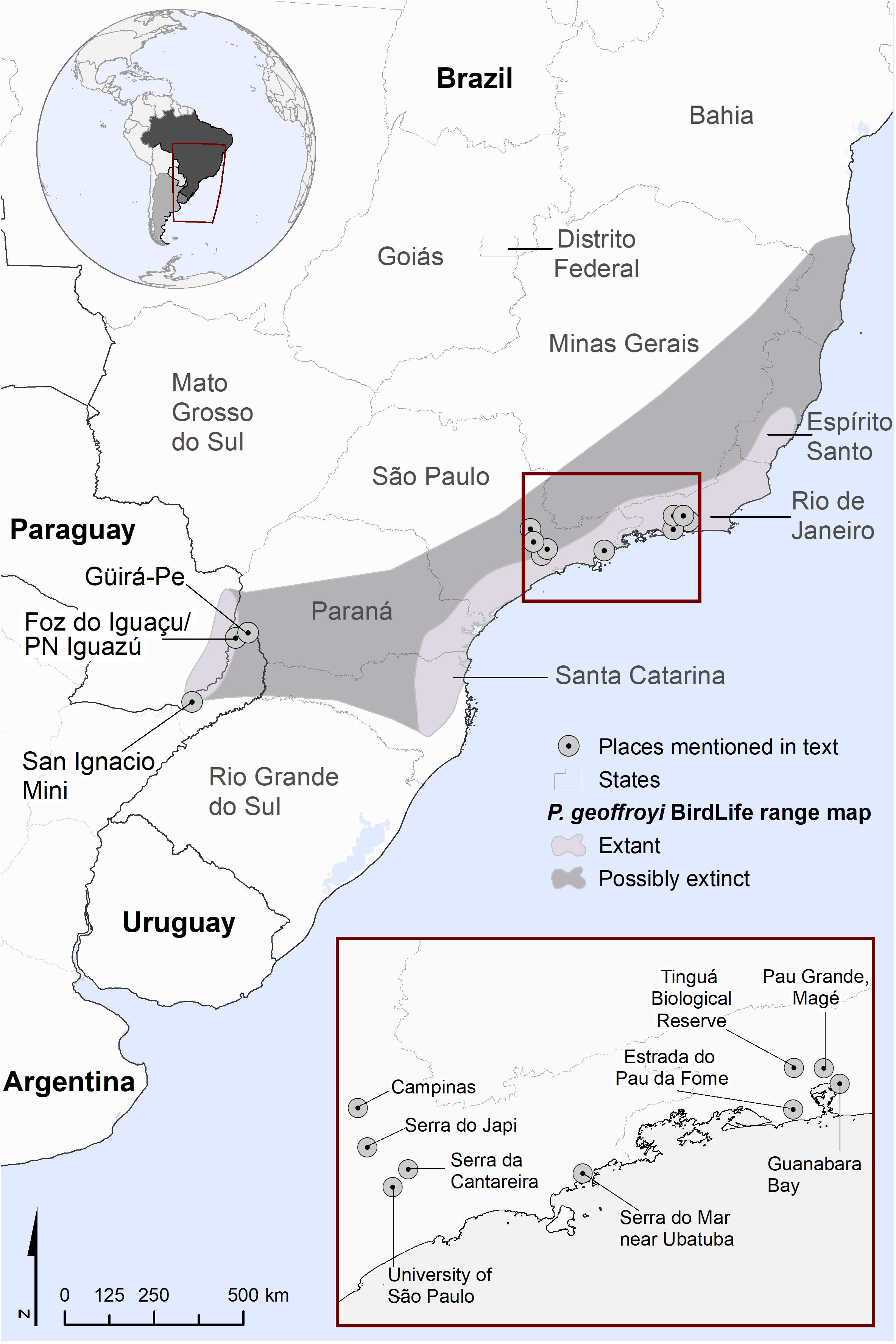
Figure 1. Distribution of Purple-winged Ground Dove according to the current range map by BirdLife International, 2021 as well as places mentioned in text, including sites of specimen records.
Herein, we develop an analysis pipeline that can be used to ascertain the persistence of “missing” bird species. We collate spatiotemporal distributional data on the Purple-winged Ground Dove derived primarily from citizen science initiatives and compared this with data available with a “control” forest pigeon of similar historical rarity which occurs in regional sympatry and examine a time series of records of variable evidence to calculate extinction likelihood. We then map all records of Purple-winged Ground Dove and build a species distribution model to identify where this species should be sought if extant. We compile citizen science image data for its most closely related allopatric species to assess possible differences in detectability. Finally, we interview bird breeders who historically kept the species frequently in captivity (King, 1978–1979), to gain insights into the species’ life history and decline, and report on the first known sound recording of the species.
Methods
Study Species
In order to ascertain the current and historical status of the Purple-winged Ground Dove, we harvested data from different sources including (1) records listed in the primary academic and gray literature resulting from targeted surveys for this species and general bird surveys, (2) specimen records (including tissue samples), (3) rich media sources (photo, video or audio) archived on citizen science initiatives or social networks, and (4) undocumented sight records from citizen science initiatives. Given that Purple-winged Ground Dove has had various taxonomic aliases over the centuries having been assigned to four genera: Columba, Peristera, Claravis, and now Paraclaravis and with two applicable species names, godefrida, and geoffroyi, we used all historically valid combinations to find references to the species (Areta et al., 2009; Sangster et al., 2018). Given the likelihood of spatiotemporal biases and changes in sampling effort, we also gathered data for another rare understorey forest-associated pigeon species occurring in regional sympatry—the Violaceous Quail-Dove (Geotrygon violacea), known from relatively few records in the Atlantic Forest region. Both taxa are skulking forest understorey species which we assume have broadly similar detection probabilities although they do have some subtle ecological differences and are not congeneric. We incorporated data into the analysis from information posted online up until December 2019. We also compiled image data for the extant, allopatric sister species of Purple-winged Ground Dove, the Maroon-chested Ground Dove (Paraclaravis mondetoura) to assess possible differences in detectability between it and Violaceous Quail-Dove, assuming that the life history and ease of detection of these two Paraclaravis species are very similar.
Records Search
To locate published historical records (see literature list in Supporting Information Table 1), we searched the Google Scholar1 and the Web of Science2 databases to locate peer-reviewed publications and grey literature citing records of these species and by checking through reference lists in each study identified. Additional (some unpublished) datasets were located based on our knowledge of historical studies and conversations with colleagues. We downloaded data for both species from eBird http://ebird.org/ and also extracted data from published records listed in, e.g., Collar et al. (1992) and Areta et al. (2009). We used the digital databases VERTNET3, specieslink4), and GBIF5 to search for pigeon specimens. We obtained data from specimens housed at the American Museum of Natural History (AMNH), Museo Argentino de Ciencias Naturales (MACN), Museu de Zoologia da Universidade de São Paulo (MZUSP), the Museu Nacional Rio de Janeiro (MNRJ), and the Museu Paraense Emílio Goeldi, Belém, Brazil (MPEG). Collecting localities were located using Paynter (1995) and Paynter and Traylor (1991). We also accessed data on banding records of both species from Argentina curated by the Centro Nacional de Anillado de Aves (CENAA)6 and requested data from Brazil held by the Centro Nacional de Pesquisa e Conservação de Aves Silvestres (CEMAVE)7.
Photographs, videos, and sound recordings, also termed rich media or digital vouchers, are important primary biodiversity records if they are diagnostic and accompanied by high-quality metadata (e.g., Lees et al., 2014). We searched for such materials using the portal iDigBio8 along with other websites dedicated to archiving bird images and sounds namely eBird/Macaulay Library9, Visual Resources for Ornithology (VIREO)10, WikiAves11, Fauna Paraguay12, and the broad repositories for the deposition of wildlife images: iNaturalist13, Project Noah14, and Discover Life15 and archival/encyclopedia sites Arkive16 and the Encyclopedia of Life17. We excluded records of sound recordings as there are no archived sound recordings of Purple-winged Ground Dove---including searching18 (although the call was described by Sick, 1997)—and so we did not include sound recordings of Violaceous Quail-Dove in the totals. We include data up to the end of the calendar year 2019.
We analyzed images from the two richest image datasets (Macaulay library and WikiAves) for Maroon-chested Ground Dove and for Violaceous Quail-Dove, to assess possible differences in detectability between the different taxa. Each photograph of these species was classified according to whether the bird(s) in it were on the ground or perched in vegetation. Our suspicion is that Violaceous Quail-Dove might spend more time on the ground and is thus likely to be more easily detected by an untargeted search, for example, when they walk on forest roads or trails. Doves perched in vegetation were assumed to be more difficult to detect unless they were vocalizing. All photos available up to October 23, 2020 were examined.
Distribution of the Purple-winged Ground Dove
To gauge range contraction, we calculated extent of occurrence (EOO), measured as a convex hull around occurrence points (IUCN, 2016) for the last two 15-year periods (approximately three generation lengths), corresponding to records post-2005 (n = 6) and for records from 1990 (n = 14). We also calculated the EOO for all records with coordinates (n = 68; Supplementary Table 1, supporting information), representing the maximum known historic range. To illustrate changing land cover dynamics within the range, we calculated habitat change within the 1990 Extent of Occurrence using land cover data from Hansen et al. (2013) and MapBiomas (MapBiomas Project, 2019). The former provides information on forest loss globally, while the latter provides more detailed land cover classes but just within Brazil. Net forest loss (Hansen et al., 2013, updated to 2019) was calculated as forest loss minus gain, starting from a baseline forest cover of >50% canopy cover in 2000 and may contain areas of plantation. The MapBiomas data was aggregated to four classes: agriculture (including pasture and plantations), forest, nonforest (including savanna, wetlands, and grasslands), and built-up (including urban areas and mining) and summed within the polygon. Calculations were made at native resolution of 30 m in Google Earth Engine for both data sets and clipped to the EOO polygon.
To identify areas to search for the species and to gauge search effort within its potential current range, we built a species distribution model following methods shown to have a higher performance for rare species with small numbers of observations (Breiner et al., 2015). Here, all possible combinations of bivariate models are built (that is, using just two predictors at a time), from which an ensemble is made, weighted by the value of Somer’s D from each bivariate model. Somer’s D, or Gini Coefficient, is a recalculation of AUC, giving more weight to models that perform well (Breiner et al., 2015). We used Maxent (Phillips et al., 2006) to build each bivariate model; it has been shown to perform well with sparse observations (as few as 10 records; Hernandez et al., 2006) and uses regularization as a form of variable selection, by shrinking predictor coefficients (Merow et al., 2013). Given the small numbers of records, we did not use hinge, threshold, or product features. We used threefold cross-validation, averaged over 10 runs, to produce accuracy metrics of AUC and Boyce’s Index (Hirzel et al., 2006). The final model was built with all occurrence points. We used R packages raster (Hijmans, 2020), ecospat (Broennimann et al., 2014), and sf (Pebesma, 2018) to perform all analyses. To focus on the current range, we included variables indicating current land cover, as well as climate and topography. Seven climate predictors, based on annual temperature and precipitation, were chosen from the suite of bioclimatic variables (bio02, bio04, bio05, bio06, bio12, bio13, bio15; Fick and Hijmans, 2017), topographic variables of elevation and slope were calculated from a digital elevation model (Jarvis et al., 2008), and current land cover was based on seasonal variation and cumulative Enhanced Vegetation Index (EVI), derived from MODIS satellite image, and combined over the period 2003–2014 (Radeloff et al., 2019). Predictors were chosen so that pairwise Pearson correlation coefficients were below 0.70 (Dormann et al., 2013). All predictors were used at a 1-km resolution. A trade-off between including sufficient observations and ensuring temporal coincidence between locality records and predictors is exacerbated with rare species. We included presence records within the last 30 years (≥ 1990). A 10% minimum omission threshold was used to produce a conservative binary map, showing the most probable area of presence. To estimate search effort and species absence, or at least nondetection, we extracted all point locations of complete eBird checklists (i.e., where bird observers have specifically noted that they were confident of recording all birds on a trip) without records of Purple-winged Ground Doves.
Ascertaining Extinction Probability
Given the exponential growth in biodiversity knowledge, it is likely becoming increasingly harder for “missing” species to evade detection, at least those for which detection and field identification in areas frequently visited by nonspecialists is an achievable goal. The record of the sightings of a species through time provides a basis for statistical inference about its possible extinction. Given the often-limited information available on many species, such methods have often concentrated on inferring extinction based on historical sighting events data (Rivadeneira et al., 2009). To infer possible extinction dates from our time series of records, we utilized the models of Strauss and Sadler (1989); Solow (1993); McInerny et al. (2006) applied using the spreadsheet of Rivadeneira et al. (2009) to estimate the upper bound (95th percentile) of the confidence interval of extinction times. We calculated this value using the most robust data (documented records, i.e., specimens) as well as our full dataset (observations plus specimens). Details about the assumptions of the methods and programming code to implement them are available in Rivadeneira et al. (2009). Additionally, we used the method provided by Thompson et al. (2017) which was also used by Butchart et al. (2018) to calculate the extinction probability cited above. Thompson’s method requires upper and lower limits of probabilities of identification (by observers) and detectability to be determined for each record (Supplementary Table 2), as well as estimating the proportion of suitable habitat within the species’ range surveyed in dedicated and passive surveys (Supplementary Table 3). Passive surveys refer to any opportunity for sighting the species in years without records that are not part of surveys specifically aimed at finding the species in question (Thompson et al., 2017). We calculated probability of being extant for each year since 1894 with data from Butchart et al. (2018) and additional data from this study. We used upper and lower limits of 0.95–0.99 for identification probability of specimen records and conservative values of 0.50–0.65 for all sight records without evidence. We used eBird absence data to estimate proportion of range covered by passive surveys by calculating the total area of 5 km buffers around eBird checklist locations within the modeled range of the species for each year (see Supplementary Tables 1–3 in supporting information for all records and input data to models). Probabilities of identification and detectability followed Butchart et al. (2018) for surveys. We projected extinction risks to 2030 by assuming no further records and that the area of passive surveys would increase linearly—a precautionary assumption, given that we have not included WikiAves image records which represent the majority of documented citizen science records of birds in Brazil (Schubert et al., 2019). We repeated the analysis considering only museum specimens.
Aviculturist Interviews
The Purple-winged Ground Dove is known to have been kept in captivity historically, and this has been suggested to have been a significant threat to the species (e.g., Collar et al., 1992). However, we are unaware of any published information regarding the ecology or husbandry of these captive birds or the reason for the demise of captive populations. We also note that the species was also absent from the list of species considered by Collar and Butchart (2014) to be appropriate for conservation-breeding programs. To understand the nature of captive populations, LFS conducted semistructured interviews with Brazilian aviculturists between 2016 and 2020. We were able to locate and personally interview most of these breeders, who provided information about the provenance of captive stock, their behavior in captivity, vocalizations, and reproduction.
Results
Overview of Records, Geographic Range, and Calculating Extinction Probability
We were able to trace 79 records of Purple-winged Ground Doves of which 49 were specimens collected between 1820 and 1985 located in 11 museum collections (Supplementary Table 1). Of these, 19 lacked date and location information. The last specimen record and hence last unambiguously documented record of the species in the wild is MNRJ#44569, a female collected on Estrada do Pau da Fome, Taquara in Rio de Janeiro, Brazil on January 15, 1985 (Figure 2A). A further female was found dead on March 15, 1991 on the campus of the University of São Paulo, now deposited at the Collection of Zoology Department (DZUSP#80); however, this may represent an escapee, given that private breeders were known to have collections at <1 km from the campus. The date also coincides with the period of the collapse of the captive breeding population (see “Discussion”). Of the 30 sight records, the most recent was in 2017 in Argentina, preceded by two sightings in 2008 in Brazil and Argentina (Figure 2A). We were unable to trace any field photographs, videos, or sound recordings of this species. Images taken by the late Luiz Cláudio Marigo of a captive bird and formerly uploaded to the defunct Arkive19 site were the sole images we were able to find from scouring internet resources. By contrast, we were able to trace 146 records of Violaceous Quail-Dove from the Atlantic Forest region of which 73 were specimens from 14 collections and 73 photographic records of the species (66 from WikiAves, 7 from the Macaulay Library), all since 2012 (Figure 2B). Both of these species were detected on a regular basis historically, whereas only Violaceous Quail-Dove has been unambiguously documented in the last 35 years. The 37,000 banded birds from Argentina include no records of either target species, while of 956,360 birds banded in Brazil, there are 29 Violaceous Quail-Doves, of which 21 were from the Atlantic Forest, and no Purple-winged Ground Doves.
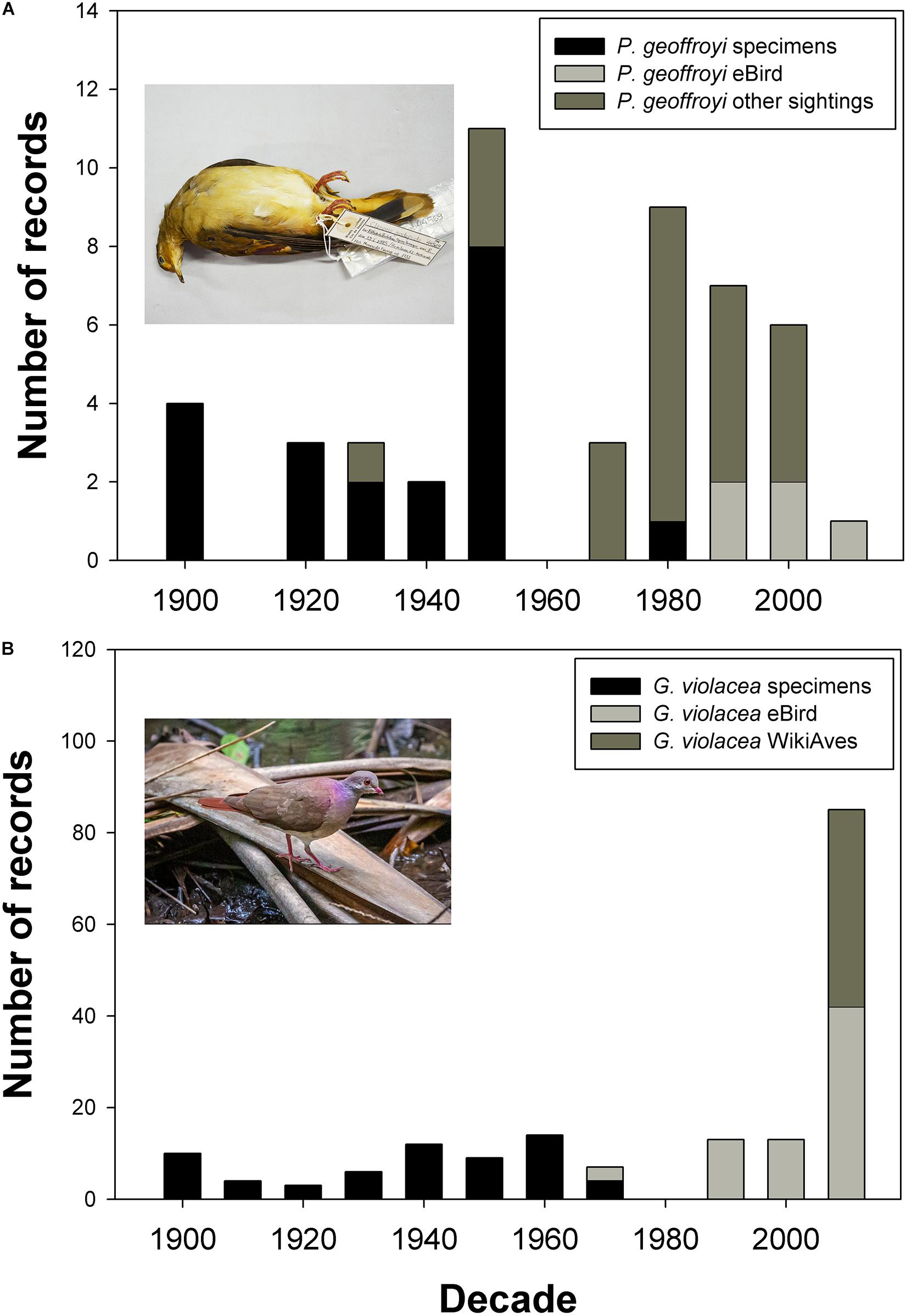
Figure 2. Temporal changes in evidence base for records of (A) Purple-winged Ground Dove Paraclaravis geoffroyi and (B) Violaceous Quail-Dove Geotrygon violacea; specimens of both species were historically collected with similar historical frequency, but only the continued presence of Violaceous Quail-Dove has been unambiguously documented in the last 35 years. Image inset in (A) is MNRJ#44569, a female Purple-winged Ground Dove collected on Estrada do Pau da Fome, Taquara in Rio de Janeiro, Brazil on 15/01/1985 (image Guilherme Brito); image inset in (B) is #WA3594946, an adult male Violaceous Quail-Dove photographed at Gália, São Paulo, Brazil on 02/12/2019 (image Paulo Fernando Bertagnolli).
Regarding detectability of Violaceous Quail-Dove, of 62 photos on eBird, 22 photos were of birds on the ground, 22 on perches, and 18 in the hand of mist-netted or injured birds. Of 73 photos of the same species in WikiAves, 17 were perched while 28 appeared to be obviously on forest tracks and many others may be adjacent to them. Of 76 photos of Maroon-chested Ground Dove, by contrast, 17 photos were of birds on the ground, and 59 on perches. For both species, some of the photos of birds on the ground were of injured birds. The ratio of males to females of Maroon-chested Ground Dove on eBird was 1.1 on the ground and 3.4 on perches, suggesting that perched birds were more likely to be detected while singing or via playback than encountered randomly without first hearing them. These findings suggest that Paraclaravis is rarely encountered except when vocalizing or attracted via playback.
Considering only the unambiguous specimen records, we obtained upper bound (95th percentile) extinction dates of 2010 (Strauss and Sadler, 1989), 2009 (Solow, 1993), and 2006 (McInerny et al., 2006) using the Rivadeneira et al. (2009) implementation, whereas the corresponding dates utilizing both specimens and sight records were 2030, 2030, and 2028. In contrast, the Thompson et al. (2017) method obtained mean probabilities of being extant today of 0.86 (0.81–0.91 ± standard deviation) for all records, and 0.67 (0.61–0.75 ± SD) for just specimen data (Figure 3). For 2030, assuming no further records come to light, projected probabilities decrease to 0.59 (0.50–0.68 ± SD) and 0.47 (0.38–0.55 ± SD) for all records and just specimens, respectively (Supplementary Tables 4,5, supporting information). The proportion of the modeled range covered by eBird checklists without positive records varied from 0.02 in 2000 to 18% in 2019. The total coverage over the last 5 years (2015–2020) amounted to 30%.
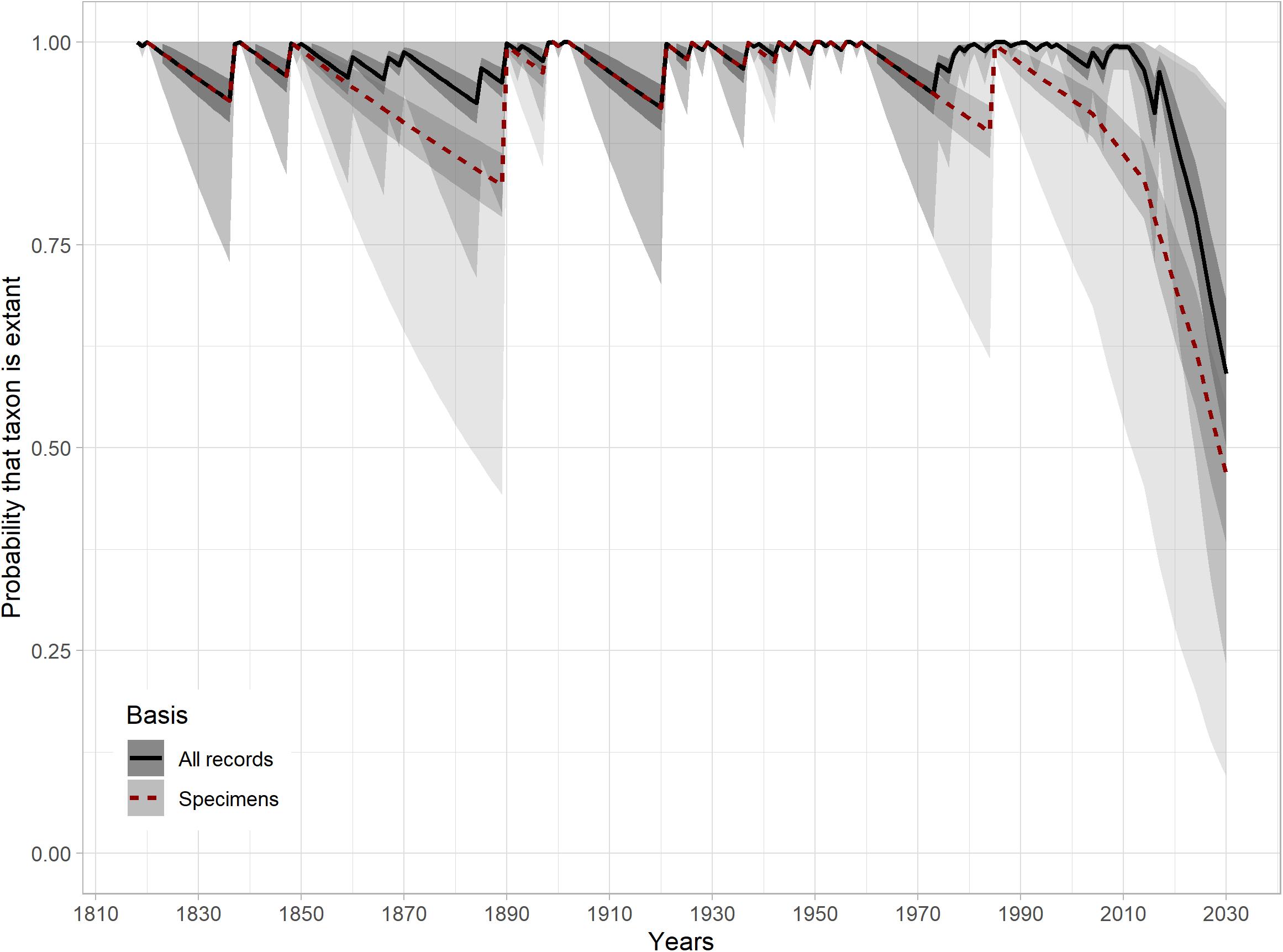
Figure 3. Probability of taxon being extant 1894–2030, for analysis using just specimen records and both specimen records and sight records. The outer gray envelopes show minimum-maximum probability estimates, whereas the inner gray envelopes show mean probability estimates ± standard deviation.
Range, Habitat Change, and Modeled Distribution
We observed an eightfold decline in the area of the EOO from the historical range to the present day. It contracted from its original range, considering all known occurrence points, of 834,642 to 277,000 km2 by 1990, and to 95,045 km2 by 2005 (Figure 4A). By this later period, all records emanated from two core regions 1) in the south-western part of the Brazilian states of São Paulo and Paraná and 2) in the Argentine province of Misiones and the Brazilian state of Paraná (Figure 4). Within the 277,000 km2 of the EOO polygon of records post-1990, 5,700 km2 of forest loss was registered between 2000 and 2012 (Hansen et al., 2013), whereas between 1990 and 2017, habitat changes just inside Brazil for this area, constituted a loss of 9,090 km2 of forest, 2,250 km2 of non-forest, and an increase in 1,050 km2 of the built environment and 9,330 km2 of agriculture (MapBiomas Project, 2019).
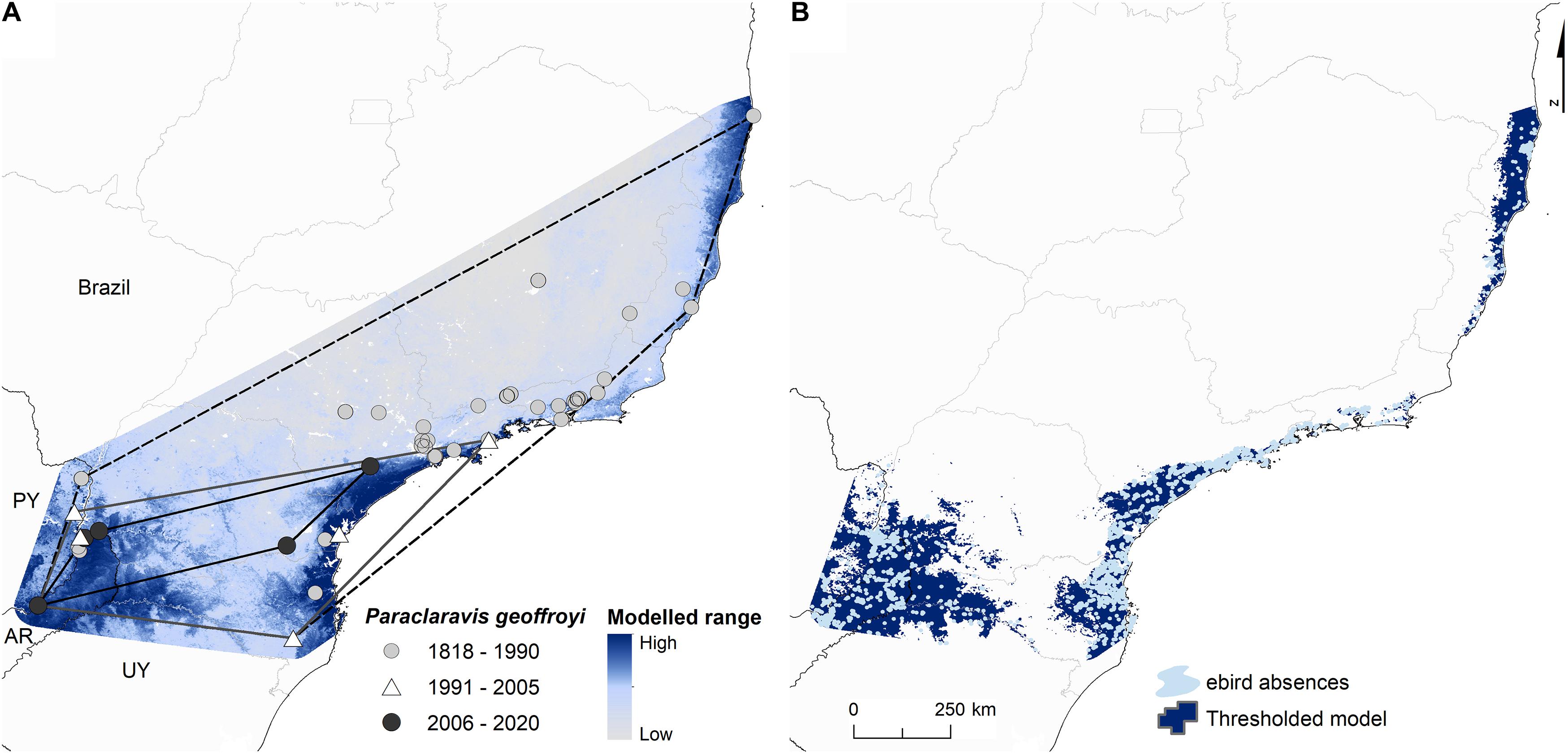
Figure 4. (A) Extent of occurrence and modeled range of Purple-winged Ground Dove, showing all known records. The dashed black line corresponds to the EOO for all records, the gray line to records 1991–2005, and the solid black line to 2006–2020. (B) Thresholded model overlaid with eBird absences, showing priority areas to conduct searches (PY, Paraguay; AR, Argentina; UY, Uruguay).
Species distributions models performed well despite the small numbers of occurrences, with mean AUC and Boyce index at 0.86 (± 0.080 SD) and 0.84 (± 0.143 SD), respectively. The model provided clear support for the obvious gap in records between the remaining coastal regions of the Atlantic Forest in Brazil and the extensive, discontinuous forests on the border of Brazil and Argentina (Figure 4A). Within the thresholded range, more than 20,000 eBird checklists from 1990 to 2020 did not locate the species, of which more than 17,900 were registered between 2011 and 2020, covering 15% of the 1-km cells of the modeled area. Priority areas to search for the species in Brazil include the east-central and extreme western parts of Santa Catarina and south-west Paraná and perhaps most important parts of Misiones in Argentina, where values of predicted occurrence are high and where the fewest eBird absences occur (Figure 4B).
History in Captivity
Between 2016 and 2020, we were able to identify and interview six former owners of Purple-winged Ground Dove from Brazil, some of whom were successful in breeding the species. Captive individuals were usually caught by chance in fall, cage-traps, or mist-nets targeting other species such as Buffy-fronted Seedeater (Sporophila frontalis) which is also associated with masting bamboo. Captured individuals were kept in community aviaries together with other doves, finches, and softbills for ornamental purposes only. In the late 1970s, a private breeder from the city of Santos, São Paulo (where the specimens were captured) initiated a captive breeding program (not underpinned by conservation goals), assigning individual aviaries to each pair of doves. Subsequently, new individuals were reported as captured in the wild in small numbers from the northern coast of São Paulo where they were captured in bamboo masting events along the foot of the Serra do Mar, about 400 m asl. In the early 1980s, another pair was obtained from Campinas, in the interior of São Paulo, also associated with masting bamboo. In the early 1980s, a further three breeders in São Paulo city successfully bred the species in captivity with varying protocols. The initial breeding stock of these three breeders all came from the same location in the Serra da Cantareira, in São Paulo, where they were apparently not associated with bamboo but were caught at the forest edge in traps baited with ripe fruits of the herb “caruru-roxo” (= Phytolacca thyrsiflora, Phytolaccaceae). CK formed a group of breeders dedicated to the dove and hence the creation of an amateur studbook to ensure that all captive individuals were paired and inbreeding was avoided. The largest breeding group, with about 70 individuals, was located at CK’s facilities comprising individuals from all breeders, including a wild-caught female obtained from a breeder in Araras, São Paulo - apparently obtained west of the city in masting bamboo. This captive breeding arrangement was expanded, with the inclusion of a breeder from Jundiaí, São Paulo, and another one from Petrópolis, Rio de Janeiro. Birds from Jundiaí may have come from the Serra do Japi, and those in Petrópolis were obtained in the 1970s and early 1980s from forests adjacent to the Biological Reserve of Tinguá, again in masting bamboo. Those responsible also reported birds from Pau Grande area, a subdistrict of Magé, Rio de Janeiro, at the foot of the Serra dos Órgãos. Apparently, the birds prefered foothill areas of the coastal slope at 400 m. The captive population reached a peak of around 150 birds.
In 1976, the Brazilian Institute of the Environment and Renewable Natural Resources (IBAMA) published the implementing regulation Portaria 031-P, obliging amateur breeders to register their birds, but forbidding breeders in Article 6 from registering any threatened species except the Large-billed Seed-Finch (Sporophila maximiliani). This situation became even more problematic for ex situ breeding efforts when in 1988 IBAMA published Portaria 131, restricting registration to passerines only. Those owning nonpasserines could keep their birds, but they could not exchange or donate them. Although the relationship between IBAMA and breeders was initially good, not all of them registered at the first opportunity, fearing restrictions on the movement of birds, and the consortium of breeders continued to send juvenile Purple-winged Ground Doves to each other. IBAMA’s policy attitudes toward breeders became progressively more hostile, viewing them as potential traffickers and imposing fines. The dove breeders group had disbanded by the late 1980s, as by then it was effectively impossible to obtain permission to send birds to breeding centers, not to mention the delay and bureaucracy involved for approval of such requests. Portaria 131, the regulation that prohibited the amateur breeding in captivity of nonpasserine birds disincentivized continued keeping of Purple-winged Ground Doves; even without it coming into law, restrictions on the transfer of birds between breeders would have led to inbreeding risks. Juveniles reared by CK were sent to IBAMA-authorized “scientific breeders,” without receiving other individuals in return, and these authorized breeders had no experience or success in the ex situ reproduction of the species. The value of the few pairs sent to zoos was unappreciated by the zoos, and no efforts were made to breed them. Although the species had been on the official Brazilian list of threatened species since 1973 (Portaria Instituto Brasileiro de Desenvolvimento Florestal No. 3.481-DN, May 31 1973) its parlous conservation status does not seem to have been widely recognized (at the time the list just had a single category). Other scientific breeders were almost nonexistent, and some doves were sent by CK to a scientific breeder in Rio de Janeiro, some of which were photographed there by Luiz Cláudio Marigo—becoming the only publicly available images of the species. CK bred ∼70 individuals but gave up on his breeding center in 1990 due to government constraints. He left the entire collection (including 39 Purple-winged Ground Doves) with a partner, who passed the remaining doves on to the IBAMA nominees. In the end, by the mid-1990s, all captive individuals had died because they were sent to those who had no practice with the idiosyncrasies of the species.
Previously unpublished images and video of captive birds held by CK in the 1980s and early 1990s (e.g., Figures 5a–c) represent the only rich media documentation of the female and juvenile plumages of the species in life and these have now been deposited with the Macaulay Library (Supplementary Table 6, audio: ML#27390020, video: ML#488757-48876521 and photos: ML#724171-72417622,23). Furthermore, a digitized video of captive Violaceous Quail-Doves includes a recording of the song of Purple-winged Ground Dove in the background—a vocalization that was hitherto unknown. We have archived a file (ML# 273900, Figure 5d) consisting of a low-quality recording of four Purple-winged Ground Dove calls, which have been edited (filtered and amplified) in the hope they will be more useful for ornithologists searching for the species either by playback or automated template matching. During the editing process, CK indicated that the call “sounded strange” as if the tape was “running at low velocity” (possibly an effect of being digitized at a slower speed than the original speed of the recorder), and that it sounded closer to his recollection when we tuned the call up by two semitones (Figure 5d). The voices of both Purple-winged Ground Dove and Maroon-chested Ground Dove are similar, with both ascending in pitch, lasting around 0.5 s, and extending across frequencies below 500 Hz (Supplementary Figure 1). The doves held by CK were kept together with Violaceous Quail-Doves, the voice of which has a longer duration (about 1 s), descends in pitch and occupies frequencies above 500 Hz.
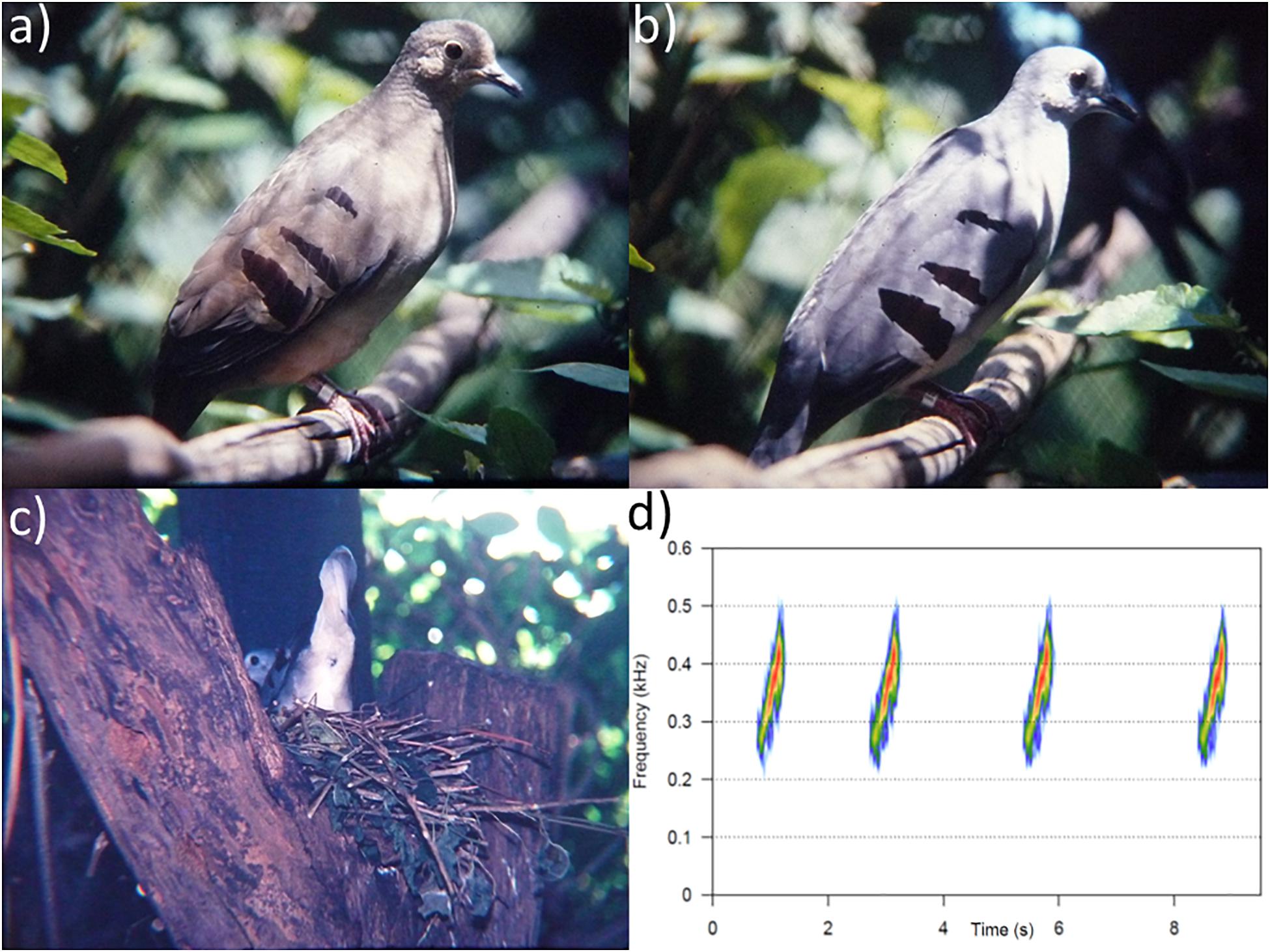
Figure 5. Portraits of captive Purple-winged Ground Doves (a) female, (b) male, and (c) incubating male on a nest at Criadouro Tropicus, Pirassununga, São Paulo in 1988, in addition to the last documented wild individual with (d) sonograms of the vocalizations of a captive Purple-winged Ground Dove obtained in 1992 ML#273900 (images (a–c) and recording by Carlos Keller).
Husbandry and Captive Breeding
The basic diet in captivity was composed of various grains, plus a mixture of seeds and finally, a small-grained pellet feed supplemented with vitamins and minerals. CK’s aviary had compact vegetation and was located in a quiet place, away from the passage of people. The doves liked to hide among foliage in the middle strata of vegetation and rarely descended to the ground; only staying in open areas for about 1 h a day to sunbathe. Although they habituated to people fairly easily, they were prone to panic—risking damage from collisions with the walls or roof of the aviary. The pairs were kept in medium-sized aviaries, about 4 m × 6 m × 3 m. The nest normally was so flimsy that the eggs were visible from underneath, and they sometimes fell through the gaps. Nesting materials provided were varied, but the doves preferred as a base some rough thin roots that could be braided without slipping, and inside the nest soft moss and some feathers. The nests were often built between thick vertical forks (Figure 5c). Two white eggs were laid, incubated by the female at night, and the male by day, changing positions silently early in the morning. The exchange was usually made with the pair positioned on the nest side by side, sliding the body laterally, so that the eggs were never exposed, perhaps as their white color might attract attention from predators. The incubation period was around 15 days and chicks used to leave the nest around the 15th day. Chicks gained independence between 18 and 20 days but often accompanied the parents for much longer until they were chased away by the male when renesting was initiated.
Discussion
We present the first attempts to model the geographic range of the Purple-winged Ground Dove, document its range contraction, describe novel aspects of its life history, and make a quantitative appraisal of its continued persistence. Our estimates of the extinction risk vary between methods, as highlighted by Rivadeneira et al. (2009). Three methods requiring only year of sighting/specimen estimate that the species would have become extinct between 2006 and 2010, considering only specimens, and by 2030, considering both sight records and specimens. The Thompson et al. (2017) method, which also requires data on detection and identification probability, as well as area surveyed, gives an earliest estimate of extinction by 2030 considering just specimens. It is likely this method is more conservative given our precautionary input probabilities applied across the board, rather than for each individual observer. However, this variation in modeled extinction dates underscores the challenge of ascertaining persistence in rare species; moreover it is not unusual for Neotropical birds to be rediscovered after disappearing for decades with no sightings (Tobias et al., 2006). There have been several such examples in eastern Brazil such as the Cherry-throated Tanager (Nemosia rourei) which was known from the type specimen and a 1941 sight record, before being rediscovered in 1998, and the Stresemann’s Bristlefront (Merulaxis stresemanni), collected around 1830, seen again in 1945 and then rediscovered in 1995. Even more extraordinarily the Kinglet Calyptura (Calyptura cristata) went unreported for 106 years between 1890 when the last specimen was collected and 1996 when there was a multi-observer record, albeit without supporting evidence in the form of images or sound recordings (Lambert and Kirwan, 2010). In the adjacent dryer biomes of the Caatinga, even larger taxa have escaped detection for decades such as Kaempfer’s Woodpecker (Celeus obrieni). In the Brazilian Cerrado, the Blue-eyed Ground Dove (Columbina cyanopis) went undocumented from 1941 until its rediscovery in 2016, outside what was believed to be its global range. However, in all these cases, these species were either extremely geographically restricted (often in remote areas), known from very few initial records/specimens, restricted to specific microhabitats (so life histories and habitat preferences are poorly known), or a combination of some or all these factors. Likewise, all the recent discoveries of new (and invariably) threatened Atlantic Forest species have involved microendemics (Lees and Pimm, 2015). These circumstances do not however apply to Purple-winged Ground Dove which was better known, and which had a large range size—within which it was encountered regularly historically.
If the species was truly nomadic, then evading detection becomes an ever more diminishing possibility as it is unlikely that the species would persist in a single unsurveyed locality in the Atlantic Forest. Even if these spots were not reached then occasional records might reasonably be expected of birds dispersing between forest patches (Areta et al., 2009; Areta and Cockle, 2012). There are historical records that suggest this behavior, with a nominally “vagrant” individual recorded as a window kill in urban São Paulo (Willis, 2000), although this might alternatively have been a bird released after regulations came in force. Beyond field observations, the Purple-winged Ground Dove also needs to have avoided other avian sampling protocols; we note that there are records of Violaceous Quail-Doves from four Brazilian municipalities (Almeida et al., 2010; Godoy, 2012; Salvadori, 2018; Gomes, 2019), including the only records on WikiAves from Bahia, which were obtained with camera traps. Camera traps are increasingly proving to be an effective means of sampling larger terrestrial bird species (O’Brien and Kinnaird, 2008) and scientists running bespoke camera trap programs should be aware of the possibility of recording these rare doves as bycatch. There have also been extensive mist-netting campaigns in the Atlantic Forest, where 21 Violaceous Quail-Dove have been banded since 1985 and this failure of mist-netting campaigns to detect Purple-winged Ground Doves is arguably a better control than field sightings which may be biased by the use of playback or the apparent greater ease of detection of the more terrestrial quail-dove. Our analysis of images and our personal field experience does suggest however that without playback or knowledge of calls, which until now were unavailable for Purple-winged Ground Dove, detectability of Paraclaravis doves is low, and even more so outside of bamboo masting events.
Given the volume of observer coverage by both professional and amateur ornithologists, there must be vanishingly few areas of suitable habitat within the Atlantic Forest that do not receive visits on an annual basis. However, we identify cold spots of low observer coverage in the Serra do Mar of Brazil, and in north-west Argentina including most of the province of Misiones, from where the most recent sightings were reported at Parque Nacional Iguazú and Güirá-Pé on the Iguazú river and at San Ignacio Miní on the Paraná river (Figure 4B). Moreover, Violaceous Quail-Doves which are also rare and local in the Atlantic Forest are still regularly photodocumented, with 44 records in the last decade alone. However, to avoid the Romeo Error (i.e., considering a species to be extinct when it is not), we suggest that search effort should be directed toward priority areas identified in Figure 4B using autonomous recorders and playback of the species voice during bamboo masting events. Indeed, searches using autonomous recorders have already begun in Foz do Iguaçu (CBA, BP). The first flowering in decades of yatevó (Guadua trinii), which occurs over several years at 30-year intervals (Parodi, 1955; Areta et al., 2009), was noted in western Paraná (Brazil) from July 2020 (CBA, BP) and it was in a seeding patch of this species that the last known sighting occurred in 2017. In the event of the discovery of any Purple-winged Ground Doves it was recommended, following a day of discussions with other ornithologists and conservationists in February 2020 (at a workshop entitled: “Purple-winged Ground Dove Claravis geoffroyi planning: making the best of good news,” report in preparation), that their eventual capture and involvement in an ex situ conservation breeding program are highly desirable, after initial observations to better understand their natural history in the wild (behavior, ecology, and movements).
The dependence of Purple-winged Ground Doves on flowering bamboo may predispose them to extinction in fragmented tropical forest landscapes (Areta et al., 2009; Areta and Cockle, 2012). Its sister species the Maroon-chested Ground Dove P. mondetoura is also a specialist of flowering bamboo along the length of the tropical Andes and in Central America and is suspected to breed semi-colonially (Blomberg et al., 2020). Declines in the Purple-winged Ground Dove population may have triggered an Allee effect resulting from decreased foraging efficiency with reduced flock size or settlement cues mediated by the presence of conspecifics to form these loose colonies (Stephens and Sutherland, 1999), reminiscent of the extinction of the Passenger Pigeon (Ectopistes migratorius) in North America - which was also a specialist on masting events, but in this case of tree nuts rather than bamboo (Novak et al., 2018).
We show that the range of Purple-winged Ground Dove contracted down to two large contiguous forest blocks in the Serra do Mar region close to São Paulo and on the Brazil-Argentina border which may have been extensive enough to support the species while it disappeared elsewhere, which supports the theory that habitat amount and degree of fragmentation are key to local persistence (Areta and Cockle, 2012). One hypothesis is that the species might have followed waves of bamboo masting events, which occur synchronously over several years at roughly 30-year intervals in single localities but are staggered over large spatial scales (Areta et al., 2009). If the dove followed masting cycles of Guadua trinii and Guadua chacoensis in Argentina and adjacent areas of Brazil, and Guadua tagoara in the Serra do Mar, it may be that movements of the species through the Atlantic Forest were impeded by the loss of almost all forest between these two remnant forest blocks, leaving a gap of several hundred kilometers. A similarly large gap was created between the Serra do Mar and areas of the potential occurrence of the species further north, in Espírito Santo and Bahia. However, fragmentation may increase chances of extinction of bamboo-seed specialists not so much by constraining connectivity, but rather by depleting the necessary alternative food sources to cope with times of bamboo-seed scarcity (Areta et al., 2009, 2013; Areta and Cockle, 2012).
The usage of clearly defined geographic ranges to understand extinction risk is routine in ascertaining extinction likelihood and our distribution models were based on this premise. However, given the inferred high mobility of Purple-winged Ground Doves and other bamboo-specialist birds, the accumulation of records over time does not provide a “range” in the traditional sense of the term, i.e., all parts of that range are not, or might never be, occupied simultaneously (Areta et al., 2013). This is critical to understanding the model map, because potential ecologically suitable areas for the presence of the species will be realistically suitable for breeding only when bamboos are masting (Areta and Cockle, 2012; Areta et al., 2013).
Given that the Purple-winged Ground Dove was bred apparently with relative ease in captivity—as early as the nineteenth century in London, England (Anon, 1878), the end of the captive population represents a major missed conservation opportunity. Clearly, the conservation community needs to avoid making this same mistake again and work with, rather than against private bird breeders (e.g., Owen et al., 2014) even if this might mean some pragmatic choices given that past collection of individuals of the species from the wild has likely had negative impacts on remaining populations. This should prove a cautionary tale for governments and conservation NGOs wrestling with the moral dilemma of dealing with breeders who may have obtained birds nefariously but who now may be the key for the survival of some species given their expert knowledge of species-specific captive husbandry.
We also draw attention to the conservation status of our “control” species, the Violaceous Quail-Dove, a species which has a widespread yet highly disjunct distribution in South and Central America but is seemingly everywhere rare; represented by only 418 observations and 33 photographs in eBird. Although the species is mapped as having a wide southern Amazonian distribution, there are fewer than 10 records (Lees et al., 2013) and the species may even be nomadic or migratory like the Ruddy Quail-Dove (Geotrygon montana) is in the same region (Stouffer and Bierregaard, 1993). By way of comparison, the more restricted range Maroon-chested Ground Dove is represented by 390 observations and 51 images on the eBird platform, which provides a useful control given that the species has a similar life history to the Purple-winged Ground Dove and is observed and photographed with a similar frequency to the partially sympatric Violaceous Quail-Dove. This suggests that it is unlikely to be prohibitively difficult to obtain field photographs of Purple-winged Ground Dove, although perhaps more difficult than the sympatric quail-dove which is more likely to be seen along quiet forest roads.
Conclusion
In conclusion, we cannot say with certainty that the Purple-winged Ground Dove still exists, nor that it is extinct. Using different methods, and placing differing emphasis on data quality, the species may have died out by the early 1990s, or it may yet persist undetected in very small numbers. One factor which impeded detection in the past was the lack of a sound recording of the species. We now have a sound recording, even if it is short and of low quality, which will contribute to the possibility of finding this species. If it still exists, the Purple-winged Ground Dove must be close to the brink of extinction and hope for its persistence will hinge on finding and capturing enough individuals to establish an ex situ insurance population, as well as intensive studies of its natural history to maximize the chances of a successful return to the wild in future. A tragic element in this story is that the species was successfully maintained and bred in captivity, but this population was lost when well-intentioned but onerous regulation created too great a bureaucratic burden on breeders, and their relationship with the regulatory authorities broke down. We are unaware of other examples of such an easily prevented extinction event in a bird species. The future for this species in the long term will depend not only on establishing an ex situ population but also on restoring functional connectivity at the landscape scale in the Atlantic Forest.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s. R code for methods can be found here: https://github.com/Cdevenish/Paraclaravis_project.
Ethics Statement
Ethical review and approval was not required for the animal study because all data was in the public domain already, no bespoke lab or field component.
Author Contributions
AL collected and curated the data. CD carried out the GIS and extinction risk analysis with input from AL. JIA performed fieldwork and surveys and CdA the sound analysis. LFS carried out the interviews with the breeders. AL wrote the first draft of the manuscript and all authors contributed to revisions.
Funding
LFS receives funds from São Paulo Research Foundation (grant no. #2017/23548-2) and the Brazilian Research Council (grant #308337/2019-0). JIA’s field searches for Purple-winged Ground Dove were made possible thanks to funding by the Preventing Extinctions Programme (BirdLife International), a Conservation Award. (Neotropical Bird Club) and a Pamela and Alexander F. Skutch Research Award for Studies in Avian Natural History (Association of Field Ornithologists), while Alejandro Bodrati, Kristina Cockle, and Ingrid Holzmann were key field companions during these field surveys.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Manuella Andrade de Souza and Diego Mendes Lima from CEMAVE for providing banding data, Jay McGowan for media archival with Macaulay Library, and Lucas Forti for initial efforts at audio filtering. Guilherme Brito and Alex Bond supplied images of specimens from MNRJ and BNHM respectively. We thank Paulo Fernando Bertagnolli for the use of the Violaceous Quail-Dove image. Paulo Flecha, Carlos Alberto Polezel Filho, Paulo Tomimori, Carlos Torloni, Alcides Vertematti, Herculano Alvarenga, Nelson Kawall (in memoriam), and Rolf Granstau (in memoriam) kindly provided information about the birds in captivity. We also thank two breeders who prefer to remain anonymous. Finally, we are also thank Matthew Bond for comments on ex situ work by private breeders in other taxa.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.624959/full#supplementary-material
Supplementary Figure 1 | Representative sonograms of the vocalizations of Purple-winged Ground Dove Paraclaravis geoffroyi, Maroon-chested Ground Dove Paraclaravis mondetoura, and Violaceous Quail-Dove Geotrygon violacea including accession numbers for recordings in Macaulay Library and xeno-canto.
Supplementary Table 1 | Records of Purple-winged Ground Dove Paraclaravis geoffroyi used in this study and associated metadata.
Supplementary Table 2 | Input data for specimen and sighting records to calculate the extinction probability of Purple-winged Ground Dove Paraclaravis geoffroyi using the method provided by Thompson et al. (2017).
Supplementary Table 3 | Field survey input data to calculate the extinction probability of Purple-winged Ground Dove Paraclaravis geoffroyi using the method provided by Thompson et al. (2017).
Supplementary Table 4 | Results of extinction risk probability estimation using specimens and sightings with the method provided by Thompson et al. (2017).
Supplementary Table 5 | Results of extinction risk probability estimation using only specimens with the method provided by Thompson et al. (2017).
Supplementary Table 6 | Accession numbers and metadata for rich media deposited at the Macaulay Library.
Footnotes
- ^ https://scholar.google.com/
- ^ https://wok.mimas.ac.uk/
- ^ http://vertnet.org/
- ^ http://splink.cria.org.br/
- ^ http://www.gbif.org/
- ^ https://www.csnat.unt.edu.ar/investigacion/institutos/cenaa/institucional/base-de-datos-de-anillado
- ^ https://www.icmbio.gov.br/cemave/
- ^ http://www.idigbio.org/
- ^ http://www.ebird.org
- ^ http://vireo.ansp.org/
- ^ http://www.wikiaves.com.br/
- ^ http://www.faunaparaguay.com
- ^ http://www.inaturalist.org/
- ^ http://www.projectnoah.org/
- ^ http://www.discoverlife.org/
- ^ https://www.wildscreen.org/arkive-closure/
- ^ http://eol.org/
- ^ https://www.xeno-canto.org/
- ^ http://arkive.org/
- ^ https://macaulaylibrary.org/asset/273900
- ^ https://ebird.org/media/catalog?taxonCode=pwgdov1&mediaType=v&cap=yes&q=Purple-winged%20Ground%20Dove%20-%20Paraclaravis%20geoffroyi
- ^ https://ebird.org/media/catalog?taxonCode=pwgdov1&mediaType=p&cap=yes&q=Purple-winged%20Ground%20Dove%20-20Paraclaravis%20geoffroyi
- ^ http://www.macaulaylibrary.org
References
Almeida, E. M. R., Toniato, M. T. Z., and Durigan, G. (2010). Plano de Manejo da Estação Ecológica de Bauru. São Paulo: Instituto Florestal. Available online at: https://smastr16.blob.core.windows.net/iflorestal/2013/03/Plano_de_Manejo_EEc_Bauru.pdf (accessed January 12, 2021).
Andermann, T. (2021). iucnsim. R-package. Available online at: https://github.com/tobiashofmann88/iucnsim
Andermann, T., Faurby, S., Cooke, R., Silvestro, D., and Antonelli, A. (2021). iucn_sim: a new program to simulate future extinctions based on IUCN threat status. Ecography 44, 162–176. doi: 10.1111/ecog.05110
Areta, J. I., Bodrati, A., and Cockle, K. (2009). Specialization on Guadua bamboo seeds by three bird species in the Atlantic Forest of Argentina. Biotropica 41, 66–73. doi: 10.1111/j.1744-7429.2008.00458.x
Areta, J. I., Bodrati, A., Thom, G., Rupp, A. E., Velazquez, M., Holzmann, I., et al. (2013). Natural history, distribution, and conservation of two nomadic Sporophila seedeaters specializing on bamboo in the Atlantic Forest. Condor 115, 237–252. doi: 10.1525/cond.2013.120064
Areta, J. I., and Cockle, K. L. (2012). A theoretical framework for understanding the ecology and conservation of bamboo-specialist birds. J. Ornithol. 153, 163–170. doi: 10.1007/s10336-012-0861-z
BirdLife International. (2021). Species factsheet: Paraclaravis geoffroyi. Available online at: http://www.birdlife.org (accessed Febrauary 25, 2021).
Blomberg, C., Greeney, H. F., and Port, J. (2020). Observations on the parental care behavior of the Maroon-chested Ground Dove (Paraclaravis mondetoura) in southeastern Ecuador. Ornitol. Neotrop. 31, 57–63.
Breiner, F. T., Guisan, A., Bergamini, A., and Nobis, M. P. (2015). Overcoming limitations of modelling rare species by using ensembles of small models. Methods Ecol. Evol. 6, 1210–1218. doi: 10.1111/2041-210x.12403
Broennimann, O., Petitpierre, B., Randin, C. F., Engler, R., Hordijk, W., Mod, H., et al. (2014). Ecospat: Spatial Ecology Miscellaneous Methods. R Package Version 1.0. Available online at: http://CRAN.R-project.org/package=ecospat (accessed February 25, 2020).
Butchart, S. H., Lowe, S., Martin, R. W., Symes, A., Westrip, J. R. S., and Wheatley, H. (2018). Which bird species have gone extinct? A novel quantitative classification approach. Biol. Conserv. 227, 9–18. doi: 10.1016/j.biocon.2018.08.014
Collar, N. J. (1998). Extinction by assumption; or, the Romeo Error on Cebu. Oryx 32, 239–244. doi: 10.1046/j.1365-3008.1998.d01-51.x
Collar, N. J., and Butchart, S. H. M. (2014). Conservation breeding and avian diversity: chances and challenges. Int. Zoo. Yearb. 48, 7–28. doi: 10.1111/izy.12039
Collar, N. J., Gonzaga, L. P., Krabbe, N., Madroño-Nieto, A., Naranjo, L. G., Parker, T. A. I. I. I., et al. (1992). Threatened Birds of the Americas. Cambridge: Smithsonian Institution Press in cooperation with the International Council for Bird Preservation.
Diamond, J. M. (1987). Extant unless proven extinct? Or, extinct unless proven extant? Conserv. Biol. 1, 77–79. doi: 10.1111/j.1523-1739.1987.tb00012.x
Dormann, C. F., Elith, J., Bacher, S., Buchmann, C., Carl, G., Carré, G., et al. (2013). Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36, 27–46. doi: 10.1111/j.1600-0587.2012.07348.x
Fick, S. E., and Hijmans, R. J. (2017). WorldClim 2: new 1km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315. doi: 10.1002/joc.5086
Fisher, D. O. (2011). Trajectories from extinction: where are missing mammals rediscovered? Glob. Ecol. Biogeogr. 20, 415–425. doi: 10.1111/j.1466-8238.2010.00624.x
Fisher, D. O., and Blomberg, S. P. (2011). Correlates of rediscovery and the detectability of extinction in mammals. Proc. R. Soc. B 278, 1090–1097. doi: 10.1098/rspb.2010.1579
Godoy, F. I. (2012). WA652404, Geotrygon violacea (Temminck, 1809)]. Wiki Aves - A Enciclopédia das Aves do Brasil. Available online at: http://www.wikiaves.com/652404 (accessed 3 April, 2021).
Gomes, P. S. (2019). WA3292717, Geotrygon violacea (Temminck, 1809)]. Wiki Aves - A Enciclopédia das Aves do Brasil. Available online at: http://www.wikiaves.com/3292717 (accessed 3 April, 2021).
Hansen, M. C., Potapov, P. V., Moore, R., Hancher, M., Turubanova, S. A., Tyukavina, A., et al. (2013). High-resolution global maps of 21st-century forest cover change. Science 342, 850–853. doi: 10.1126/science.1244693
Hernandez, P. A., Graham, C. H., Master, L. L., and Albert, D. L. (2006). The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 29, 773–785. doi: 10.1111/j.0906-7590.2006.04700.x
Hijmans, R. J. (2020). Raster: Geographic Data Analysis and Modeling. (3.0-12) [R]. Available online at: http://CRAN.R-project.org/package=raster (accessed February 25, 2020).
Hirzel, A. H., Le Lay, G., Helfer, V., Randin, C. F., and Guisan, A. (2006). Evaluating the ability of habitat suitability models to predict species presences. Ecol. Model. 199, 142–152. doi: 10.1016/j.ecolmodel.2006.05.017
IUCN (2016). Guidelines for Using the IUCN Red List Categories and Criteria. Version 12.0. IUCN Standards and Petitions Working Group. Available online at: http://www.iucnredlist.org/documents/RedListGuidelines.pdf (accessed 12 Oct 2020)
Jarvis, A., Reuter, H. A., Nelson, A., and Guevara, E. (2008). Hole-Filled Seamless SRTM data V4 [Map]. International Centre for Tropical Agriculture. Available online at: http://srtm.csi.cgiar.org (accessed October 12, 2020).
King, W. B. (1978–1979). Red Data Book, 2. Aves, Second Edn. Morges: International Union for Conservation of Nature and Natural Resource.
Lambert, F., and Kirwan, G. M. (2010). The twice-vanishing ‘pardalote’: what future for the Kinglet Calyptura? Neotrop. Birding 6, 4–17.
Lees, A. C., Naka, L. N., Aleixo, A., Cohn-Haft, M., de Piacentini, V. Q., Santos, M. P. D., et al. (2014). Conducting rigorous avian inventories: amazonian case studies and a roadmap for improvement. Rev. Bras. Ornitol. 22, 107–120. doi: 10.1007/bf03544240
Lees, A. C., and Pimm, S. L. (2015). Species, extinct before we know them? Curr. Biol. 25, R177–R180.
Lees, A. C., Zimmer, K. J., Marantz, C. A., Whitaker, A., Davis, B. J. W., and Whitney, B. M. (2013). Alta Floresta revisited: an updated review of the avifauna of the most intensively surveyed locality in south-central Amazonia. Bull. Brit. Ornithol. Club 133, 178–239.
MapBiomas Project (2019). Project MapBiomas - Brazilian Land Cover & Use Map Series (Collection 3.1) [Map]. MapBiomas Project. Available online at: https://mapbiomas.org (accessed February 25, 2020).
Mazar Barnett, J., and Buzzetti, D. R. C. (2014). A new species of Cichlocolaptes Reichenbach 1853 (Furnariidae), the ‘gritador-do-nordeste’, an undescribed trace of the fading bird life of northeastern Brazil. Ornithol. Res. 22, 75–94. doi: 10.1007/bf03544237
McInerny, G. J., Roberts, D. L., Davy, A. J., and Cribb, P. J. (2006). Significance of sighting rate in inferring extinction and threat. Conserv. Biol. 20, 562–567. doi: 10.1111/j.1523-1739.2006.00377.x
Merow, C., Smith, M. J., and Silander, J. A. (2013). A practical guide to MaxEnt for modeling species’ distributions: what it does, and why inputs and settings matter. Ecography 36, 1058–1069. doi: 10.1111/j.1600-0587.2013.07872.x
MMA (2014). Lista Nacional Oficial de Espécies da Fauna Ameaçadas de Extinção. Portaria No 444, de 17 de dezembro de 2014. Diário Oficial da União - Seção 1. N° 245, Quinta-Feira, 18 December 2014.
Newbold, T. (2010). Applications and limitations of museum data for conservation and ecology, with particular attention to species distribution models. Prog. Phys. Geogr. 34, 3–22. doi: 10.1177/0309133309355630
Novak, B. J., Estes, J. A., Shaw, H. E., Novak, E. V., and Shapiro, B. (2018). Experimental investigation of the dietary ecology of the extinct passenger pigeon, Ectopistes migratorius. Front. Ecol. Evol. 6:20. doi: 10.3389/fevo.2018.00020
O’Brien, T. G., and Kinnaird, M. F. (2008). A picture is worth a thousand words: the application of camera trapping to the study of birds. Bird Conserv. Int. 18(S1), S144–S162.
Owen, A., Wilkinson, R., and Sözer, R. (2014). In situ conservation breeding and the role of zoological institutions and private breeders in the recovery of highly endangered Indonesian passerine birds. Int. Zoo. Yearb. 48, 199–211. doi: 10.1111/izy.12052
Parodi, L. R. (1955). La floración de la tacuara brava (“Guadua trinii”). Rev. Arg. Agron 22, 134–136.
Paynter, R. A. (1995). Ornithological Gazetteer of Argentina. Obtainable from Bird Department, Museum of Comparative Zoology. Cambridge, MA: Harvard University.
Paynter, R. A., and Traylor, M. A. (1991). Ornithological Gazetteer of Brazil. Mus. Comp. Zool. Cambridge, MA: Harvard University Press.
Pebesma, E. (2018). Simple features for R: standardized support for spatial vector data. R J. 1, 439–446. doi: 10.32614/rj-2018-009
Phillips, S., Anderson, R., and Schapire, R. (2006). Maximum entropy modeling of species geographic distributions. Ecol. Modell. 190, 231–259. doi: 10.1016/j.ecolmodel.2005.03.026
Radeloff, V. C., Dubinin, M., Coops, N. C., Allen, A. M., Brooks, T. M., Clayton, M. K., et al. (2019). The dynamic habitat indices (DHIs) from MODIS and global biodiversity. Remote Sens. Environ. 222, 204–214. doi: 10.1016/j.rse.2018.12.009
Rivadeneira, M. M., Hunt, G., and Roy, K. (2009). The use of sighting records to infer species extinctions: an evaluation of different methods. Ecology 90, 1291–1300. doi: 10.1890/08-0316.1
Salvadori, J. P. (2018). WA3351158, Geotrygon violacea (Temminck, 1809)]. Wiki Aves - A Enciclopédia das Aves do Brasil. Available online at: http://www.wikiaves.com/3351158 (accessed October 9, 2020).
Sangster, G., Sweet, A. D., and Johnson, K. P. (2018). Paraclaravis, a new genus for the Purple-winged and Maroon-chested Ground-doves (Aves: Columbidae). Zootaxa 446, 134–140. doi: 10.11646/zootaxa.4461.1.10
Scheffers, B. R., Yong, D. L., Harris, J. B. C., Giam, X., and Sodhi, N. S. (2011). The world’s rediscovered species: back from the brink? PLoS One 6:e22531. doi: 10.1371/journal.pone.0022531
Schubert, S. C., Manica, L. T., and Guaraldo, A. D. C. (2019). Revealing the potential of a huge citizen-science platform to study bird migration. Emu 119, 364–373. doi: 10.1080/01584197.2019.1609340
Solow, A. R. (1993). Inferring extinction in a declining population. J. Math. Biol. 32, 79–82. doi: 10.1007/bf00160376
Stephens, P. A., and Sutherland, W. J. (1999). Consequences of the Allee effect for behaviour, ecology and conservation. Trends Ecol. Evol. 14, 401–405. doi: 10.1016/s0169-5347(99)01684-5
Stouffer, P. C., and Bierregaard, R. O. Jr. (1993). Spatial and temporal abundance patterns of Ruddy Quail-Doves (Geotrygon montana) near Manaus, Brazil. Condor 95, 896–903. doi: 10.2307/1369427
Strauss, D., and Sadler, P. M. (1989). Classical confidence intervals and Bayesian probability estimates for ends of local taxon ranges. Math. Geol. 21, 411–427. doi: 10.1007/bf00897326
Thompson, C. J., Koshkina, V., Burgman, M. A., Butchart, S. H. M., and Stone, L. (2017). Inferring extinctions II: a practical, iterative model based on records and surveys. Biol. Conserv. 214, 328–335. doi: 10.1016/j.biocon.2017.07.029
Tobias, J. A., Butchart, S. H., and Collar, N. J. (2006). Lost and found: a gap analysis for the Neotropical avifauna. Neotrop. Bird 1, 4–22.
Keywords: sighting record, extinction, citizen science, avian conservation, deforestation
Citation: Lees AC, Devenish C, Areta JI, de Araújo CB, Keller C, Phalan B and Silveira LF (2021) Assessing the Extinction Probability of the Purple-winged Ground Dove, an Enigmatic Bamboo Specialist. Front. Ecol. Evol. 9:624959. doi: 10.3389/fevo.2021.624959
Received: 01 November 2020; Accepted: 05 March 2021;
Published: 29 April 2021.
Edited by:
Bruktawit Abdu Mahamued, Kotebe Metropolitan University (KMU), EthiopiaReviewed by:
John Woinarski, Charles Darwin University, AustraliaSam Turvey, Zoological Society of London, United Kingdom
Copyright © 2021 Lees, Devenish, Areta, de Araújo, Keller, Phalan and Silveira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexander C. Lees, alexander.lees@mmu.ac.uk
 Alexander C. Lees
Alexander C. Lees Christian Devenish
Christian Devenish Juan Ignacio Areta
Juan Ignacio Areta Carlos Barros de Araújo4,5
Carlos Barros de Araújo4,5  Ben Phalan
Ben Phalan Luís Fábio Silveira
Luís Fábio Silveira