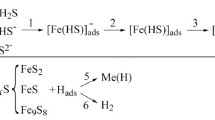
We investigate the corrosion-electrochemical properties of 17G1SU steel in chloride-acetate solutions for different concentrations of hydrogen sulfide. It is shown that, if this concentration increases, then the corrosion potential of steel shifts to the side of more negative values and the corrosion rate increases almost linearly. The presence of hydrogen sulfide does not affect the values of Tafel constants of the cathodic and anodic reactions. It is shown that the rate of cathodic reactions may become higher as a result of the direct reduction of hydrogen from H2S molecules. For hydrogen-sulfide concentrations > 1500 mg/dm3, the rate of cathodic processes decreases due to the complications of diffusion, which may be caused by the adsorption of hydrogen-sulfide molecules and a decrease in the rate of catalytic recombination of adsorbed hydrogen atoms. Hydrogen-induced cracking of stress-free steel is revealed for hydrogen-sulfide concentrations ≥ 500 mg/dm3.


Similar content being viewed by others
References
I. V. Plachkov and S. H. Plachkova, Power Engineering: History, State-of the-Art, and Future [in Ukrainian, Electron resource]; Access mode: http://energetika.in.ua/ua/books/book-1.
G. Koch, J. Varney, N. Thompson, O. Moghissi, M. Gould, and J. Payer, “NACE 2016 impact study” [Electron resource] (2016), pp. 1–16. Access mode: http://impact.nace.org/documents/summary-supplement.pdf.
Laboratory Testing of Metals for Resistance to Sulfide Stress Cracking and Stress Corrosion Cracking in H2S, NACE Standard TM0177-2005, NACE Int. (2005), pp. 1–39.
М. S. Khoma, S. А. Holovei, V. R. Ivashkiv, M. R. Chuchman, and Ch. В. Vasyliv, “Effect of sulfides on the hydrogen overvoltage and hydrogenation of U8 steel in chloride-hydrogen-sulfide media,” Fiz.-Khim. Mekh. Mater., 53, No. 6, 16–21 (2017); English translation: Мater. Sci., 53, No. 6, 761–768 (2018).
L. I. Antropov, Theoretical Electrochemistry [in Ukrainian], Lybid’, Kiev (1993).
D. E. Jiang and E. A. Carter, “First principles study of H2S adsorption and dissociation on Fe(110),” Аррl. Surf. Sci., 583, 60–68 (2005).
D. Rickard and G. W. Luther, “Chemistry of iron sulfides,” Chem. Rev., 107, 514–562 (2007).
H. Ma, X. Cheng, G. Li, S. Chen, Z. Quan, S. Zhao, and L. Niu, “The influence of hydrogen sulfide on corrosion of iron under different conditions,” Corr. Sci., 42, 1669–1683 (2000).
J. Ning, Y. Zheng, D. Young, B. Brown, and S. Nešic, “Thermodynamic study of hydrogen sulfide corrosion of mild steel,” Corrosion, 70, No. 4, 375–389 (2008).
D. Rickard, “Kinetics of fast precipitation reactions involving metal sulfides,” Chem. Geol., 70, 81 (1988).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Fizyko-Khimichna Mekhanika Materialiv, Vol. 56, No. 4, pp. 100–104, July–August, 2020.
Rights and permissions
About this article
Cite this article
Khoma, М.S., Іvashkiv, V.R., Ratska, N.B. et al. Corrosion-Electrochemical Properties of 17G1SU Steel in Chloride-Acetate Solutions with Different Concentrations of Hydrogen Sulfide. Mater Sci 56, 544–549 (2021). https://doi.org/10.1007/s11003-021-00462-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11003-021-00462-0