Abstract
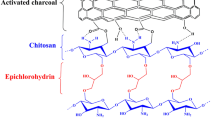
Composite materials have elicited much interest because of their superior performance in the removal of toxic and radioactive uranyl ions from aqueous solutions. With polyethyleneimine as a functional group, carboxylated chitosan as a matrix, and oxidizing activated carbon as a nanofiller, this study synthesized a novel environment-friendly polyethylenimine-functionalized carboxylated chitosan/oxidized activated charcoal (PCO) biocomposite with a unique three-dimensional porous structure. PCO was synthesized through an easy chemical cross-linking method. Detailed characterization certified the formation of the unique three-dimensional porous structure. The obtained PCO was used to remove uranyl ions from an aqueous solution, demonstrating the maximum adsorption capacity of 450 mg·g−1. The adsorption capacity of PCO decreased by less than 7.51% after five adsorption-desorption cycles. PCO exhibited good adsorption selectivity (Kd = 3.45 × 104 mL·g−1) for uranyl ions. The adsorption mechanism of PCO was also discussed. The material showed good potential for application in the treatment of wastewater containing uranyl ions.

Similar content being viewed by others
References
Xu H, Cao C S, Hu H S, Wang S B, Liu J C, Cheng P, Kaltsoyannis N, Li J, Zhao B. High uptake of ReO4- and CO2 conversion by a radiation-resistant thoriumnickle [Th48Ni6] nanocage-based metalorganic framework. Angewandte Chemie International Edition, 2019, 58(18): 6022–6027
Tabasum S, Noreen A, Maqsood M F, Umar H, Akram N, Nazli Z I, Chatha S A S, Zia K M. A review on versatile applications of blends and composites of pullulan with natural and synthetic polymers. International Journal of Biological Macromolecules, 2018, 120: 603–632
Ao J X, Yuan Y H, Xu X, Xu L, Xing Z, Li R, Wu G Z, Guo X J, Ma H J, Li Q N. Trace zinc-preload for enhancement of uranium adsorption performance and antifouling property of AO-functionalized UHMWPE fiber. Industrial & Engineering Chemistry Research, 2019, 58(19): 8026–8034
Huang S, Jiang S, Pang H, Wen T, Asiri A M, Alamry K A, Alsaedi A, Wang X, Wang S. Dual functional nanocomposites of magnetic MnFe2O4 and fluorescent carbon dots for efficient U(VI) removal. Chemical Engineering Journal, 2019, 368: 941–950
Li B, Sun Q, Zhang Y, Abney C W, Aguila B, Lin W, Ma S. Functionalized porous aromatic framework for efficient uranium adsorption from aqueous solutions. ACS Applied Materials & Interfaces, 2017, 9(14): 12511–12517
Liu H, Zhou Y, Yang Y, Zou K, Wu R, Xia K, Xie S. Synthesis of polyethylenimine/graphene oxide for the adsorption of U(VI) from aqueous solution. Applied Surface Science, 2019, 471: 88–95
Ladshaw A, Kuo L J, Strivens J, Wood J, Schlafer N, Yiacoumi S, Tsouris C, Gill G. Influence of current velocity on uranium adsorption from seawater using an amidoxime-based polymer fiber adsorbent. Industrial & Engineering Chemistry Research, 2017, 56(8): 2205–2211
Zhan W, Xu C, Qian G, Huang G, Tang X, Lin B. Adsorption of Cu (II), Zn(II), and Pb(II) from aqueous single and binary metal solutions by regenerated cellulose and sodium alginate chemically modified with polyethyleneimine. RSC Advances, 2018, 8(33): 18723–18733
Sun X, Chen J H, Su Z, Huang Y, Dong X. Highly effective removal of Cu(II) by a novel 3-aminopropyltriethoxysilane functionalized polyethyleneimine/sodium alginate porous membrane adsorbent. Chemical Engineering Journal, 2016, 290: 1–11
Su S, Chen R, Liu Q, Liu J, Zhang H, Li R, Zhang M, Liu P, Wang J. High efficiency extraction of U(VI) from seawater by incorporation of polyethyleneimine, polyacrylic acid hydrogel and Luffa cylindrical fibers. Chemical Engineering Journal, 2018, 345: 526–535
Dai Z, Sun Y, Zhang H, Ding D, Li L. Highly efficient removal of uranium(VI) from wastewater by polyamidoxime/polyethyleneimine magnetic graphene oxide. Journal of Chemical & Engineering Data, 2019, 64(12): 5797–5805
Lu Y, Wang Z, Ouyang X K, Ji C, Liu Y, Huang F, Yang L Y. Fabrication of cross-linked chitosan beads grafted by polyethylenimine for efficient adsorption of diclofenac sodium from water. International Journal of Biological Macromolecules, 2020, 145: 1180–1188
Kim G M, Wang Z, Kang S B, Won S W. Polyethylenimine-crosslinked chitin flake as a biosorbent for removal of Acid Blue 25. Korean Journal of Chemical Engineering, 2019, 36(9): 1455–1465
Anbinder P S, Macchi C, Amalvy J, Somoza A. A study of the structural changes in a chitosan matrix produced by the adsorption of copper and chromium ions. Carbohydrate Polymers, 2019, 222: 114987
Saleh T A, Naeemullah, Tuzen M, Sarı A. Polyethylenimine modified activated carbon as novel magnetic adsorbent for the removal of uranium from aqueous solution. Chemical Engineering Research & Design, 2017, 117: 218–227
Xie X, Gao H, Luo X, Su T, Zhang Y, Qin Z. Polyethyleneimine modified activated carbon for adsorption of Cd(II) in aqueous solution. Journal of Environmental Chemical Engineering, 2019, 7(3): 103183
Wang S, Vincent T, Faur C, Rodríguez Castellón E, Guibal E. A new method for incorporating polyethyleneimine (PEI) in algal beads: high stability as sorbent for palladium recovery and supported catalyst for nitrophenol hydrogenation. Materials Chemistry and Physics, 2019, 221: 144–155
Cao F, Shen J. PEI-modified CMKGM/GO porous biocomposite for superior removal of Pb(II). Journal of Chemical & Engineering Data, 2019, 64(12): 5622–5629
Cheng Y, He P, Dong F, Nie X, Ding C, Wang S, Zhang Y, Liu H, Zhou S. Polyamine and amidoxime groups modified bifunctional polyacrylonitrile-based ion exchange fibers for highly efficient extraction of U(VI) from real uranium mine water. Chemical Engineering Journal, 2019, 367: 198–207
Shi P F, Zhao B, Xiong G, Hou Y L, Cheng P. Fast capture and separation of, and luminescent probe for, pollutant chromate using a multi-functional cationic heterometal-organic framework. Chemical Communications, 2012, 48(66): 8231–8233
Godiya C B, Liang M, Sayed S M, Li D, Lu X. Novel alginate/polyethyleneimine hydrogel adsorbent for cascaded removal and utilization of Cu2+ and Pb2+ ions. Journal of Environmental Management, 2019, 232: 829–841
Li J, Si X, Li X, Wang N, An Q, Ji S. Preparation of acid-resistant PEI/SA composite membranes for the pervaporation dehydration of ethanol at low pH. Separation and Purification Technology, 2018, 192: 205–212
Meng Q, Liu J, Jiang Y, Teng Q. Branched polyethyleneiminefunctionalized polystyrene resin: preparation and adsorption of Cu2+. Journal of Chemical & Engineering Data, 2019, 64(6): 2618–2626
Sadergaski L R, Stoxen W, Hixon A E. Uranyl peroxide nanocluster (U60) persistence and sorption in the presence of hematite. Environmental Science & Technology, 2018, 52(5): 3304–3311
Al-Harahsheh M, AlJarrah M, Mayyas M, Alrebaki M. High-stability polyamine/amide-functionalized magnetic nanoparticles for enhanced extraction of uranium from aqueous solutions. Journal of the Taiwan Institute of Chemical Engineers, 2018, 86: 148–157
Xia X, Shen J, Cao F, Wang C, Tang M, Zhang Q, Wei S. A facile synthesis of hydroxyapatite for effective removal strontium ion. Journal of Hazardous Materials, 2019, 368: 326–335
Pang H, Huang S, Wu Y, Yang D, Wang X, Yu S, Chen Z, Alsaedi A, Hayat T, Wang X. Efficient elimination of U(VI) by polyethyleneimine decorated fly ash. Inorganic Chemistry Frontiers, 2018, 5(10): 2399–2407
Su S, Liu Q, Liu J, Zhang H, Li R, Jing X, Wang J. Polyethyleneimine-functionalized Luffa cylindrica for efficient uranium extraction. Journal of Colloid and Interface Science, 2018, 530: 538–546
Wang X, Liu Q, Liu J, Chen R, Zhang H, Li R, Li Z, Wang J. 3D self-assembly polyethyleneimine modified graphene oxide hydrogel for the extraction of uranium from aqueous solution. Applied Surface Science, 2017, 426: 1063–1074
Zhu W, Dang Q, Liu C, Yu D, Chang G, Pu X, Wang Q, Sun H, Zhang B, Cha D. Cr(VI) and Pb(II) capture on pH-responsive polyethyleneimine and chloroacetic acid functionalized chitosan microspheres. Carbohydrate Polymers, 2019, 219: 353–367
Kumar D, Gaur J P. Chemical reaction- and particle diffusion-based kinetic modeling of metal biosorption by a Phormidium sp.-dominated cyanobacterial mat. Bioresource Technology, 2011, 102(2): 633–640
Lv Z, Wang H, Chen C, Yang S, Chen L, Alsaedi A, Hayat T. Enhanced removal of uranium(VI) from aqueous solution by a novel Mg-MOF-74-derived porous MgO/carbon adsorbent. Journal of Colloid and Interface Science, 2019, 537: A1–A10
Bayramoglu G, Akbulut A, Arica M Y. Study of polyethyleneimine- and amidoxime-functionalized hybrid biomass of Spirulina (Arthrospira) platensis for adsorption of uranium(VI) ion. Environmental Science and Pollution Research International, 2015, 22(22): 17998–18010
Huang G, Li W, Liu Q, Liu J, Zhang H, Li R, Li Z, Jing X, Wang J. Efficient removal of uranium(VI) from simulated seawater with hyperbranched polyethylenimine (HPEI)-functionalized polyacrylonitrile fibers. New Journal of Chemistry, 2018, 42(1): 168–176
Wang P, Yin L, Wang J, Xu C, Liang Y, Yao W, Wang X, Yu S, Chen J, Sun Y, Wang X. Superior immobilization of U(VI) and 243 Am(III) on polyethyleneimine modified lamellar carbon nitride composite from water environment. Chemical Engineering Journal, 2017, 326: 863–874
El Maghrabi H H, Younes A A, Salem A R, Rabie K, El Shereafy E S. Magnetically modified hydroxyapatite nanoparticles for the removal of uranium(VI): preparation, characterization and adsorption optimization. Journal of Hazardous Materials, 2019, 378: 120703
Christou C, Philippou K, Krasia-Christoforou T, Pashalidis I. Uranium adsorption by polyvinylpyrrolidone/chitosan blended nanofibers. Carbohydrate Polymers, 2019, 219: 298–305
Yan Y, An Q, Xiao Z, Zheng W, Zhai S. Flexible core-shell/beadlike alginate@PEI with exceptional adsorption capacity, recycling performance toward batch and column sorption of Cr(VI). Chemical Engineering Journal, 2017, 313: 475–486
Zhang J, Zhang H, Liu Q, Song D, Li R, Liu P, Wang J. Diaminomaleonitrile functionalized double-shelled hollow MIL-101 (Cr) for selective removal of uranium from simulated seawater. Chemical Engineering Journal, 2019, 368: 951–958
Acknowledgements
This work was financially supported by the basic research project of Sichuan Province for Science and Technology Development (Grant No. 2019YJ0355), Outstanding Youth Science and Technology Talents Program of Sichuan (Grant No. 19JCQN0085), Key Projects of the Pre-research Fund of the General Armament Department (Grant No. 6140720020101) and National Defense Technology Foundation Project (Grant No. JSJL2016404B002).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
11705_2021_2054_MOESM1_ESM.pdf
Effective and selective adsorption of uranyl ions by porous polyethylenimine-functionalized carboxylated chitosan/oxidized activated charcoal composite
Rights and permissions
About this article
Cite this article
Shen, J., Cao, F., Liu, S. et al. Effective and selective adsorption of uranyl ions by porous polyethylenimine-functionalized carboxylated chitosan/oxidized activated charcoal composite. Front. Chem. Sci. Eng. 16, 408–419 (2022). https://doi.org/10.1007/s11705-021-2054-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11705-021-2054-x