Abstract
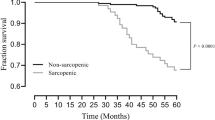
Patients with chronic kidney disease (CKD) are at increased risks of both sarcopenia and fragility fractures. However, information on the association between skeletal muscle mass (SMM) and the risk of bone fractures in patients with CKD is lacking. We performed a cross-sectional analysis of 4146 patients with CKD using the baseline dataset of the Fukuoka Kidney disease Registry Study, as a multicenter, prospective cohort study of pre-dialysis CKD patients. The main measure was estimated SMM (eSMM) calculated using an equation validated by bioelectrical impedance analysis with two independent datasets of 100 and 81 CKD patients. The main outcome was historical bone fractures. The associations between sex-specific quartiles (Q1–Q4) of eSMM and fracture history were assessed by logistic regression analyses. The prevalence of a history of fractures increased and eSMM decreased with progressive CKD stages. Among the 4146 patients, 249 had prior bone fractures, including 111 patients in Q1 (lowest quartile), 65 in Q2, 46 in Q3, and 27 in Q4 (highest quartile). A multivariable-adjusted model revealed that patients in Q1 had a significantly higher odds ratio (95% confidence interval) for bone fracture history than those in Q4 (reference): Q1, 2.77 (1.32–5.80); Q2, 1.95 (1.05–3.65); and Q3, 1.57 (0.90–2.75) (P-value for trend < 0.001). Similar associations were obtained when other skeletal muscle surrogates were applied: serum creatinine to serum cystatin C and daily urinary creatinine excretion. These results suggest that a lower eSMM is associated with an increased prevalence of historical bone fractures in pre-dialysis CKD patients.




Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Code used for the statistical analysis during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Nickolas TL, McMahon DJ, Shane E (2006) Relationship between moderate to severe kidney disease and hip fracture in the United States. J Am Soc Nephrol 17(11):3223–3232
Tentori F et al (2014) High rates of death and hospitalization follow bone fracture among hemodialysis patients. Kidney Int 85(1):166–173
Wakasugi M, Kazama JJ, Narita I (2014) High rates of death and hospitalization follow bone fracture among hemodialysis patients. Kidney Int 86(3):649
Alem AM et al (2000) Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int 58(1):396–399
Stehman-Breen CO et al (2000) Risk factors for hip fracture among patients with end-stage renal disease. Kidney Int 58(5):2200–2205
Maravic M et al (2014) Incidence and risk factors for hip fractures in dialysis patients. Osteoporos Int 25(1):159–165
Whitlock RH et al (2019) The Fracture Risk Assessment Tool (FRAX®) predicts fracture risk in patients with chronic kidney disease. Kidney Int 95(2):447–454
Wilson LM et al (2017) Benefits and harms of osteoporosis medications in patients with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med 166(9):649–658
Jamal SA (2011) Effects of denosumab on fracture and bone mineral density by level of kidney function. J Bone Miner Res 26(8):1829–1835
Connelly K, Collister D, Tangri N (2018) Fracture risk and treatment in chronic kidney disease. Curr Opin Nephrol Hypertens 27(3):221–225
Fahal IH (2014) Uraemic sarcopenia: aetiology and implications. Nephrol Dial Transplant 29(9):1655–1665
Pereira RA et al (2015) Sarcopenia in chronic kidney disease on conservative therapy: prevalence and association with mortality. Nephrol Dial Transplant 30(10):1718–1725
Souza VA et al (2017) Sarcopenia in patients with chronic kidney disease not yet on dialysis: analysis of the prevalence and associated factors. PLoS ONE 12(4):e0176230
Enoki Y et al (2017) Potential therapeutic interventions for chronic kidney disease-associated sarcopenia via indoxyl sulfate-induced mitochondrial dysfunction. J Cachexia Sarcopenia Muscle 8(5):735–747
Zhou Y et al (2018) Sarcopenia and relationships between muscle mass, measured glomerular filtration rate and physical function in patients with chronic kidney disease stages 3–5. Nephrol Dial Transplant 33(2):342–348
Tran J et al (2019) Gait abnormalities and the risk of falls in CKD. Clin J Am Soc Nephrol 14(7):983–993
Labriola L, Jadoul M (2018) Fractures in CKD patients: action plans should not overlook the prevention of falls! Kidney Int 93(5):1247
Kutner NG, Bowling CB (2019) Targeting fall risk in CKD. Clin J Am Soc Nephrol 14(7):965–966
Goto NA et al (2020) The association between chronic kidney disease, falls, and fractures: a systematic review and meta-analysis. Osteoporos Int 31(1):13–29
Tanaka S et al (2017) The Fukuoka Kidney disease Registry (FKR) Study: design and methods. Clin Exp Nephrol 21(3):465–473
Kawasaki T et al (1993) A simple method for estimating 24 h urinary sodium and potassium excretion from second morning voiding urine specimen in adults. Clin Exp Pharmacol Physiol 20(1):7–14
Kawasaki T et al (1991) Prediction of 24-hour urinary creatinine excretion from age, body weight and height of an individual and its application. Nihon Koshu Eisei Zasshi 38(8):567–574
Kashani KB et al (2017) Evaluating muscle mass by using markers of kidney function: development of the sarcopenia index. Crit Care Med 45(1):e23–e29
Lin YL et al (2020) Serum creatinine to cystatin C ratio predicts skeletal muscle mass and strength in patients with non-dialysis chronic kidney disease. Clin Nutr 39(8):2435–2441
Matsuo S et al (2009) Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53(6):982–992
Horio M et al (2013) GFR estimation using standardized serum cystatin C in Japan. Am J Kidney Dis 61(2):197–203
Kaji H (2013) Linkage between muscle and bone: common catabolic signals resulting in osteoporosis and sarcopenia. Curr Opin Clin Nutr Metab Care 16(3):272–277
Maurel DB, Jähn K, Lara-Castillo N (2017) Muscle-bone crosstalk: emerging opportunities for novel therapeutic approaches to treat musculoskeletal pathologies. Biomedicines 5(4):62
Kirk B, Zanker J, Duque G (2020) Osteosarcopenia: epidemiology, diagnosis, and treatment-facts and numbers. J Cachexia Sarcopenia Muscle 11(3):609–618
Kim S et al (2014) The association between the low muscle mass and osteoporosis in elderly Korean people. J Korean Med Sci 29(7):995–1000
Papageorgiou M, Sathyapalan T, Schutte R (2019) Muscle mass measures and incident osteoporosis in a large cohort of postmenopausal women. J Cachexia Sarcopenia Muscle 10(1):131–139
Bettis T, Kim BJ, Hamrick MW (2018) Impact of muscle atrophy on bone metabolism and bone strength: implications for muscle-bone crosstalk with aging and disuse. Osteoporos Int 29(8):1713–1720
Yamada S et al (2017) Modified creatinine index and the risk of bone fracture in patients undergoing hemodialysis: the Q-Cohort Study. Am J Kidney Dis 70(2):270–280
Kiel DP et al (2001) Bone loss and the progression of abdominal aortic calcification over a 25 year period: the Framingham Heart Study. Calcif Tissue Int 68(5):271–276
Stojanovic OI et al (2011) Association between atherosclerosis and osteoporosis, the role of vitamin D. Arch Med Sci 7(2):179–188
Anagnostis P et al (2009) Atherosclerosis and osteoporosis: age-dependent degenerative processes or related entities? Osteoporos Int 20(2):197–207
Zofková I (2008) Hormonal aspects of the muscle-bone unit. Physiol Res 57(Suppl 1):S159-169
Ji LL, Kang C (2015) Role of PGC-1α in sarcopenia: etiology and potential intervention - a mini-review. Gerontology 61(2):139–148
Miyamoto T et al (2011) Circulating follistatin in patients with chronic kidney disease: implications for muscle strength, bone mineral density, inflammation, and survival. Clin J Am Soc Nephrol 6(5):1001–1008
Wen MS et al (2013) Decrease in irisin in patients with chronic kidney disease. PLoS ONE 8(5):e64025
Schoenau E (2005) From mechanostat theory to development of the “Functional Muscle-Bone-Unit.” J Musculoskelet Neuronal Interact 5(3):232–238
Cianferotti L, Brandi ML (2014) Muscle-bone interactions: basic and clinical aspects. Endocrine 45(2):165–177
Wood CL (2017) Osteocyte secreted factors inhibit skeletal muscle differentiation. Bone Rep 6:74–80
Koppe L, Fouque D, Kalantar-Zadeh K (2019) Kidney cachexia or protein-energy wasting in chronic kidney disease: facts and numbers. J Cachexia Sarcopenia Muscle 10(3):479–484
Koh JM et al (2005) Higher circulating hsCRP levels are associated with lower bone mineral density in healthy pre- and postmenopausal women: evidence for a link between systemic inflammation and osteoporosis. GS. Osteoporos Int 16(10):1263–1271
Acknowledgements
We would like to thank all the doctors and medical staff who participated in the FKR Study.
Steering Committee and Principal Collaborators of the FKR Study Group: Satoru Fujimi (Fukuoka Renal Clinic), Hideki Hirakata (Fukuoka Renal Clinic), Tadashi Hirano (Hakujyuji Hospital), Tetsuhiko Yoshida (Hamanomachi Hospital), Takashi Deguchi (Hamanomachi Hospital), Hideki Yotsueda (Harasanshin Hospital), Kiichiro Fujisaki (Iizuka Hospital), Keita Takae (Japanese Red Cross Fukuoka Hospital), Koji Mitsuiki (Japanese Red Cross Fukuoka Hospital), Akinori Nagashima (Japanese Red Cross Karatsu Hospital), Ritsuko Katafuchi (Kano Hospital), Hidetoshi Kanai (Kokura Memorial Hospital), Kenji Harada (Kokura Memorial Hospital), Tohru Mizumasa (Kyushu Central Hospital), Takanari Kitazono (Kyushu University), Toshiaki Nakano (Kyushu University), Toshiharu Ninomiya (Kyushu University), Kumiko Torisu (Kyushu University), Akihiro Tsuchimoto (Kyushu University), Shunsuke Yamada (Kyushu University), Hiroto Hiyamuta (Kyushu University), Shigeru Tanaka (Kyushu University), Dai Matsuo (Munakata Medical Association Hospital), Yusuke Kuroki (National Fukuoka-Higashi Medical Center), Hiroshi Nagae (National Fukuoka-Higashi Medical Center), Masaru Nakayama (National Kyushu Medical Center), Kazuhiko Tsuruya (Nara Medical University), Masaharu Nagata (Shin-eikai Hospital), Taihei Yanagida (Steel Memorial Yawata Hospital), Shotaro Ohnaka (Tagawa Municipal Hospital). We also thank Susan Furness, PhD, from Edanz Group (https://en-author-services.edanzgroup.com/ac) for editing a draft of this manuscript.
Funding
There is nothing to be declared.
Author information
Authors and Affiliations
Contributions
Conception and study design: SY, ST; data acquisition: ST, HH, HA, and KT; data analysis interpretation: SY, ST, HA, HH, MT; statistical analysis: SY and ST; supervision or mentorship: TN, KT, and TK. Each author contributed important intellectual content during manuscript drafting and accepts accountability for the overall work by ensuring that questions related to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. KT takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no financial and non-financial interest regarding the present paper.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
All authors gave consent for publication to the journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yamada, S., Tanaka, S., Arase, H. et al. Associations Between Surrogates of Skeletal Muscle Mass and History of Bone Fracture in Patients with Chronic Kidney Disease: The Fukuoka Kidney disease Registry (FKR) Study. Calcif Tissue Int 109, 393–404 (2021). https://doi.org/10.1007/s00223-021-00851-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-021-00851-2