Abstract
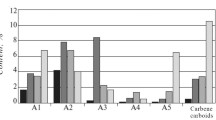
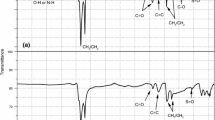
A combination of analytical methods (including CHNS-O elemental analysis, X-ray diffraction, 1H and 13C NMR spectroscopy, MALDI-TOF and ICP mass spectrometry) was used to determine the elemental composition and average molecular and structural parameters of asphaltenes isolated from a heavy oil sample from the Ashalchinskoye field and vacuum residue samples from two different Russian oil refineries. The study identified differences in the nano-aggregate size of asphaltenes in heavy oil and vacuum residues, and evaluated the effects of the composition and molecular structure of asphaltenes on the composition and properties of the heavy petroleum feedstock samples.
Similar content being viewed by others
REFERENCES
Sergienko, S.R., Taimova, B.A., and Talalaev, E.I., Vysokomolekulyarnye neuglevodorodnye soedineniya nefti (High Molecular Weight Non-Hydrocarbon Compounds of Oil), Moscow: Nauka, 1979.
Tukhvatullina, A.Z., Barskaya, E.E., Kouryakov, V.N., Ganeeva, Yu.M., Yusupova, T.N., and Romanov, G.V., J. Pet. Environ. Biotechnol., 2013, vol. 4, no. 4, pp. 1–8. https://doi.org/10.4172/2157-7463.1000152
Honse, S.O., Mansur, C.R.E., and Lucas, E.F., J. Braz. Chem. Soc., 2012, vol. 23, no. 12, pp. 2204–2210. https://doi.org/10.1590/S0103-50532013005000002
Akbarzadeh, K., Hammami, A., Kharrat, A., Zhang, D., Creek, J., Kabir, S., Jamaluddin, A.J., Marshall, A.G., Rodgers, R.P., Mullins, O.C., and Solbakken, T., Oilfield Rev., 2007, vol. 19, no. 2, pp. 22–43.
Mullins, O.C. and Sheu, E.Y., Structure and Dynamics of Asphaltenes, New York: Springer, 1998.
Ganeeva, Yu.M., Yusupova, T.N., and Romanov, G.V., Russ. Chem. Rev., 2011, vol. 80, no. 10, pp. 993–1008. https://doi.org/10.1070/RC2011v080n10ABEH004174
Mullins, O.C., Ann. Rev. Anal. Chem., 2011, vol. 4, no. 1, pp. 393–418. https://doi.org/10.1146/annurev-anchem-061010-113849
Mullins, O.C., Energy Fuels, 2010, vol. 24, no. 4, pp. 2179–2207. https://doi.org/10.1021/ef900975e
Sedghi, M., Goual, L., Welch, W., and Kubelka, J., J. Phys. Chem. B, 2013, vol. 117, no. 18, pp. 5765–5776. https://doi.org/10.1021/jp401584u
Ramirez-Corredores, M.M., The Science and Technology of Unconventional Oils: Finding Refining Opportunities, London: Academic Press, 2017.
Fan, M., Sun, X., Xu, Z., Zhao, S., Xu, C., and Chung, K.H., Energy Fuels, 2011, vol. 25, no. 7, pp. 3060–3067. https://doi.org/10.1021/ef2003359
Ariza, E., Chaves-Guerrero, A., and Molina, V.D., Energy Fuels, 2018, vol. 32, no. 6, pp. 6557–6564. https://doi.org/10.1021/acs.energyfuels.8b00664
ASTM D6560-12 (IP 143) – Standard test method for determination of asphaltenes (heptane insolubles) in crude petroleum and petroleum products, ASTM International, 2012.
ASTM D189-14 – Standard test method for Conradson carbon residue of petroleum products, ASTM International, 2014.
IP 469/01-2006 Determination of saturated, aromatic and polar compounds in petroleum products by thin layer chromatography and flame ionization detection, Energy Institute (Institute of Petroleum), 2006.
Xia, W., Xu, T., and Wang, H., J. Hazard. Mater., 2019, vol. 373, pp. 741–752. https://doi.org/10.1016/j.jhazmat.2019.04.004
Liu, Y.-J. and Li, Z.-F., J. Chem., 2015, vol. 2015, pp. 1–8. https://doi.org/10.1155/2015/580950
Michael, G., Al-Siri, M., Khan, Z.H., and Ali, F.A., Energy Fuels, 2005, vol. 19, no. 4, pp. 1598–1605. https://doi.org/10.1021/ef049854l
Ali, F.A., Ghaloum, N., and Hauser, A., Energy Fuels, 2006, vol. 20, no. 1, pp. 231–238. https://doi.org/10.1021/ef050130z
AlHumaidan, F.S., Hauser, A., Rana, M.S., Lababidi, H.M.S., and Behbehani, M., Fuel, 2015, vol. 150, pp. 558–564. https://doi.org/10.1016/j.fuel.2015.02.076
Christopher, J., Sarpal, A.S., Kapur, G.S., Krishna, A., Tyagi, B.R., Jain, M.C., Jain, S.K., and Bhatnagar, A.K., Fuel, 1996, vol. 75, no. 8, pp. 999–1008. https://doi.org/10.1016/0016-2361(96)00023-3
Yen, T.F., Erdman, J.G., and Pollack, S.S., Anal. Chem., 1961, vol. 33, no. 11, pp. 1587–1594. https://doi.org/10.1021/ac60179a039
Gray, M.R., Tykwinski, R.R., Stryker, J.M., and Tan, X., Energy Fuels, 2011, vol. 25, no. 7, pp. 3125–3134. https://doi.org/10.1021/ef200654p
Krayushkin, V.A., Guseva, E.E., and Morozova, R.M., Geol. Zh., 2008, no. 4, pp. 26–38.
Speight, J.G., Oil Gas Sci. Technol., 2004, vol. 59, no. 5, pp. 467–477. https://doi.org/10.2516/ogst:2004032
Bansal, V., Patel, M.B., and Sarpal, A.S., Petrol. Sci. Technol., 2004, vol. 22, nos. 11–12, pp. 1401–1426. https://doi.org/10.1081/lft-200027776
Zheng, C., Zhu, M., and Zhang, D., Energy Proc., 2017, vol. 105, pp. 143–148. https://doi.org/10.1016/j.egypro.2017.03.293
Magomedov, R.N., Pripakhaylo, A.V., and Maryutina, T.A., Russ. J. Phys. Chem. B, 2020, vol. 14, no. 7, pp. 1098– 1102. https://doi.org/10.1134/S1990793120070131
Funding
The study described here was performed with financial support from the Russian Foundation for Basic Research (research project no. 18-29-06044 mk).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest requiring disclosure in this article.
Additional information
Translated from Neftekhimiya, 2021, Vol. 61, No. 3, pp. 328–336 https://doi.org/10.31857/S0028242121030047.
Rights and permissions
About this article
Cite this article
Panyukova, D.I., Magomedov, R.N., Savonina, E.Y. et al. Effects of the Composition and Molecular Structure of Asphaltenes on the Properties of Heavy Petroleum Feedstock Represented by Heavy Oil from the Ashalchinskoye Field and Two Vacuum Residue Samples. Pet. Chem. 61, 438–445 (2021). https://doi.org/10.1134/S0965544121050108
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544121050108