Abstract
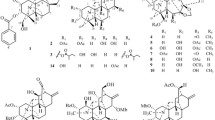
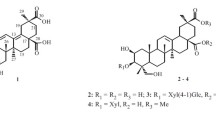
From the leaves of Ardisia quinquegona, two alkylated tetronic acid derivatives, named ardisiatetrons A and B (1, 2), and four triterpenoids (3–6) were isolated together with one known compound (7) by a combination of various kinds of chromatography. The structure of new methyl migrated triterpene (3) was confirmed by X-ray crystallographic analysis. Compounds 2, 3, and 7 showed moderate anti-Leishmania activity and cytotoxicity towards A549 cells.
Graphic abstract








Similar content being viewed by others
References
Hatusima S (1975) Flora of the Ryukyus. Added and corrected. The Biological Society of Okinawa, Naha, p 375
Zhong X-N, Otsuka H, Ide T, Hirata E, Takushi A, Takeda Y (1997) Three flavonol glycosides from leaves of Myrsine sequinii. Phytochemistry 46:943–946
Zhong X-N, Ide T, Otsuka H, Hirata E, Takeda Y (1998) (+)-Isolarisiresinol 3a-O-sulpahte from leaves of Myrsine seguinii. Phytochemistry 49:1777–1778
Zhong X-N, Otsuka H, Ide T, Hirata E, Takushi A, Takeda Y (1998) Hydroquinone glycoside from leaves of Myrsine seguinii. Phytochemistry 49:2149–2153
Zhong X-N, Otsuka H, Ide T, Hirata E, Takeda Y (1999) Hydroquinone diglycosides acyl esters from the leaves of Myrsine seguinii. Phytochemistry 52:923–927
Otsuka H, Zhong X-N, Hirata ST, Takeda Y (2001) Myrsinosionosides A–E: megastigmane glycosides from the leaves of Myrsine seguinii. Chem Pharm Bull 49:1093–1097
Matsunami K, Otsuka H, Takeda Y (2011) Myriseguinosides A–E, five new glycosides from the fruits of Myrssine seguinii. Chem Pharm Bull 59:1274–1280
Asaumi S, Kawakami S, Sugimoto S, Matsunami K, Otsuka H, Shinzato T (2018) Alkylated benzoquinones: ardisiaquinones A–H from the leaves of Ardisia quinquegona and their anti-Leishmania acrivity. Chem Pharm Bull 66:757–763
Asaumi S, Kawakami S, Sugimoto S, Matsunami K, Otsuka H, Shinzato T (2019) Alkylated benzoquinones: quinquequinones A–H from the leaves of Ardisia quinquegona and their anti-Leishmania activity. Chem Pharm Bull 67:79
Dar AA, Dangroo NA, Raina A, Qayum A, Singh S, Kumar A, Sangwan PL (2016) Biologically active xanthones from Codonopsis ovata. Phytochemistry 132:102–108
Ibraheim ZZ (2002) Triterpenes from Rubia cordiflora. Bull Pharm Sci Assuit Univ 25:155–164
Bourguet-Kondracki M-L, Guyot M (1999) A new sesquiterpene tetronic acid derivatives from the marine sponge, Smenospongia sp. Tetrahedron Lett 40:3149–3150
Parsons IC, Gray AI, Waterman PG (1993) New triterpenes and flavonoids from the leaves of Bosistoa brassii. J Nat Prod 56:46–53
Januário AH, Vieira PC, Fernandes da Silva MFDG, Fernandes JB, de Brito Silva JJ, Conserva LM (2009) Alcaloides β-indolopiridoquinzolínicos de Esenbeckia grandiflora Mart. (Rutaceae). Quim Nova 32:2034–2038
Sheldrick GM (1996) SADABS. University of Göttingen, Göttingen
Sheldrick GM (2014) SHELXT-integrated space-group and crystal structure determination. Acta Crystallogr A 71:3–8
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Crystallogr C 71:3–8
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Method 65:55–63
Acknowledgements
The authors are grateful for access to the mass spectrometer, an Applied Biosystem QSTAR XL system ESI (Nano Spray)-MS at the Analysis Center of Life Science of the Graduate School of Biomedical Sciences, Hiroshima University. This work was supported in part by Grants-in-Aid from the Ministry of Education, Science, Sports, Culture and Technology of Japan, and the Japan Society for the Promotion of Science (Nos. 22590006, 23590130, and 15H04651).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kawakami, S., Ishinaka, M., Asaumi, S. et al. Ardisiatetrons A and B: tetronic acid derivatives and triterpenes from the leaves of Ardisia quinquegona and their biological activity. J Nat Med 75, 643–654 (2021). https://doi.org/10.1007/s11418-021-01513-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-021-01513-1