Abstract
One of the most frequent symptoms of COVID-19 is the loss of smell and taste. Based on the lack of expression of the virus entry proteins in olfactory receptor neurons, it was originally assumed that the new coronavirus (severe acute respiratory syndrome coronavirus 2, SARS-CoV-2) does not infect olfactory neurons. Recent studies have reported otherwise, opening the possibility that the virus can directly infect the brain by traveling along the olfactory nerve. Multiple animal models have been employed to assess mechanisms and routes of brain infection of SARS-CoV-2, often with conflicting results. We here review the current evidence for an olfactory route to brain infection and conclude that the case for infection of olfactory neurons is weak, based on animal and human studies. Consistent brain infection after SARS-CoV-2 inoculation in mouse models is only seen when the virus entry proteins are expressed abnormally, and the timeline and progression of rare neuro-invasion in these and in other animal models points to alternative routes to the brain, other than along the olfactory projections. COVID-19 patients can be assured that loss of smell does not necessarily mean that the SARS-CoV-2 virus has gained access to and has infected their brains.
Similar content being viewed by others
Introduction
It is now well established that nearly half of all patients with COVID-19 have a reduction or loss of smell as one of their symptoms [97], resulting in tens of millions of cases of—for the most part transiently—reduced smell. Since some viruses can be “neuro-invasive,” meaning that they can enter the nervous system, there has been a concern that the new coronavirus, SARS-CoV-2, may reach the brain, using the nose as a portal, as is known or suspected for a subset of other viruses [33, 73, 95]. Is there convincing evidence that SARS-CoV-2 can infect olfactory neurons and can travel along their axons from the nose to the brain? It is known that—in rare cases—SARS-CoV-2 is present in the human brain [34, 65, 66, 68, 75, 86, 88], and it was suggested that infection of respiratory centers in the brainstem may contribute to fatal outcomes in COVID-19 [5, 31, 40, 54, 58, 63, 64, 89]. Since a large number of patients with COVID-19 lose their sense of smell, do all these people have to live in fear about a subsequent brain infection, as recent provocative titles of publications would suggest: “Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19” [66] and “SARS-CoV-2 invades the central nervous system via the olfactory route in Rhesus monkeys” [51]? In this review, we critically evaluate the current evidence whether SARS-CoV-2 may utilize olfactory neurons as a route to brain infection.
Can olfactory neurons become infected by SARS-CoV-2?
Because of the high viral load in the nasal epithelium [48, 66, 83, 100, 107] and because of the proximity of the nasal cavity to the skull and brain, many investigators have considered the possibility that SARS-CoV-2 travels from the nose to the brain along the olfactory nerve [10, 14,15,16, 18, 19, 59, 66, 72, 81, 108], similar to some of the other neuro-invasive viruses [29, 50, 89]. The cellular and molecular consequences of SARS-CoV-2 infection in the olfactory epithelium have now been examined in increasing detail, in multiple animal models as well as in human cell and tissue samples obtained by brush sampling, through biopsy, and post-mortem analysis (Tables 1, 2 and 3).
To assess whether olfactory receptor neurons may be susceptible to infection by SARS-CoV-2, investigators have determined which cell types in the olfactory epithelium express the obligatory entry proteins for the new coronavirus, angiotensin-converting enzyme 2 (ACE2) and transmembrane protease, serine 2 (TMPRSS2). These gene and protein expression studies were performed by RNAseq of identified cell types, or using markers for distinct cell types within the olfactory epithelium combined with gene or protein expression for ACE2 and TMPRSS2 [6, 8, 11, 23, 38, 42, 52, 66, 98, 103, 106]. The large majority of these studies concluded that sustentacular cells (the primary support cells in the olfactory epithelium) and cells in Bowman’s glands express the virus entry proteins, while all human studies and the majority of animal studies reported that olfactory receptor neurons do not express ACE2, or express ACE2 only very rarely (Tables 1, 2 and 3).
Another series of studies examined where in the olfactory epithelium the new coronavirus accumulates, by employing in situ hybridization or antibodies against viral proteins in histological sections [15, 20, 28, 66, 87, 98, 102, 103] or by brush sampling [28]. Some of these studies conducted double-labeling with antibodies against viral proteins as well as antibodies for specific cell types in the olfactory epithelium [15, 20, 28, 66, 87, 98, 103]. While the data of most of these studies show that the sustentacular cells are the main type of cells accumulating the virus, consistent with the predictions of the virus entry protein studies, some investigators reported that the virus can also be found in olfactory receptor neurons [28, 35, 66, 87, 98, 103]. Whether olfactory neurons become infected is an important question because of the possibility of axonal transport of the virus from the nose into the brain. Neuro-invasive viruses that use the olfactory route are known to bind with high affinity to olfactory receptor neurons [33, 56, 95].
Explanations for contradictory reports on neuron infection and neuro-invasion
How can the contradictory findings between studies on the expression of entry virus proteins and several of the virus-localization studies be reconciled? Does the virus indeed accumulate in olfactory receptor neurons (and their axons), and not only in the sustentacular cells and gland cells? Important peculiarities of the olfactory system may explain why different studies have arrived at different conclusions.
-
1.
The olfactory epithelium contains millions of olfactory receptor neurons and sustentacular cells. Most of the studies reporting an infection of olfactory neurons by SARS-CoV-2 do not provide a quantitative analysis. They describe few examples of putative olfactory neurons containing SARS-CoV-2 and display high magnification images of the olfactory epithelium showing isolated olfactory neurons possibly co-labeled for SARS-CoV-2. Studies that examined SARS-CoV-2 distribution semi-quantitatively showed that the virus mostly localizes to sustentacular cells and Bowman’s gland cells [15, 57, 98], while olfactory neurons do not contain SARS-CoV-2 or contain it only rarely [15, 98, 103, 104]. The only proper quantification so far was made in human ACE2 transgenic mice [98], and the authors found only 0.03% of mature olfactory receptor neurons to be infected.
-
2.
Virus-infected olfactory epithelium has been shown to contain dying neurons. Some of these dying neurons are phagocytosed by immune cells [28], but they can also be phagocytosed by sustentacular cells [85, 92], ensuring the removal of receptor neurons that die due to constant turnover [53]. Accordingly, some sustentacular cells will contain phagocytosed proteins that are normally found only in olfactory neurons, possibly including neuronal markers. This can create false positives, because the viral protein of an infected sustentacular cell that phagocytosed a neuron or neuronal debris may appear as an example of a cell containing viral protein co-localized with a neuronal marker protein, even when the dying neuron downregulates such marker proteins. Indeed, virus-infected olfactory epithelium has been shown to contain dying neurons (recognized by their fragmented nuclei and chromatin condensation), some of which were being phagocytosed [28]. Accordingly, occasional co-localization of neuronal and viral proteins may generate false positives.
-
3.
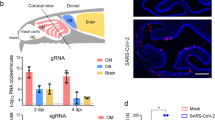
Sustentacular support cells tightly wrap olfactory receptor neurons, and especially their dendrites extending towards the nasal cavity [13, 60, 61]. This makes it difficult to distinguish between protein content within the neuronal compartment and protein content within the support cell compartment [98]. Accordingly, at a superficial glance and by merging confocal images at the light-microscopic level, the two labels may appear to overlap, when they actually are in distinct spaces, just very close together. This is illustrated in Fig. 1 adapted from the work of Bryche et al. [15] where it can be seen that only one cell, a sustentacular cell, contains the virus (Fig. 1a, red label), and not the adjacent neuron, but this is apparent only when the top of the labeled sustentacular cell is visible within the same tissue section (Fig. 1a)—if the section had been cut too thin or anything less than perfectly perpendicular to the plane of the epithelium, the virus would have been deemed to be located within the neuron (Fig. 1b). The entwinement of olfactory neurons with their support cell may explain why some studies reported viral protein in olfactory neurons, when the viral protein in fact may have been present in the tight wrappings of sustentacular cells [28, 35, 66, 87, 98, 103].
-
4.
Examination at the electron microscopic level presents an alternative approach to avoid the false positive evaluation that may arise from fluorescent-based localization of cell type-specific markers. Only two studies have explored the cellular localization of the virus in the olfactory epithelium with this technique [28, 66]. However, both studies may have misinterpreted their images. Ciliated respiratory cells differ from ciliated dendritic knobs of olfactory receptor neurons at the ultrastructural level (see, e.g., [36], their Fig. 3a, c). Fig. 3c–f in Meinhardt et al. [66] shows virus protein in such a ciliated cell, not in an olfactory neuron’s ciliated dendritic knob. Furthermore, the arrows in Fig. 3a in [66] may indicate the luminal portions of sustentacular cells, not a dendritic knob, because the size of one of these “knobs” is incompatible with the known size of knobs (they are about 1–3 µm in diameter [37, 52]), as we and others have previously pointed out [7, 19, 24]. Accordingly, current evidence for SARS-CoV-2 or viral protein in the dendrites of olfactory receptor neurons is questionable. Similarly, some error seems to have been made in the interpretation of respiratory and olfactory epithelium [28]. Their Fig. 4b and e display respiratory ciliated cells which are interpreted as belonging to the olfactory epithelium.
-
5.
In the few studies that describe SARS-CoV-2 localized in olfactory receptor neurons, the virus appears to be more often localized in immature rather than mature olfactory receptor neurons, consistent with the lack of ACE2 expression in mature olfactory neurons [5, 24] (Tables 1, 2 and 3). However, as explained in detail below, the immature neurons do not yet have axons that extend to their target glomerulus in the olfactory bulb [53, 61, 62, 85]. Therefore, when their cell bodies were infected in the olfactory epithelium, they would not be able to carry the virus to the olfactory bulb. Even if SARS-CoV-2 persists in such neurons until they are mature, the time required for maturation far exceeds the time at which the virus arrives in the brain in animal models.
-
6.
It is interesting that most of the virus-containing axons shown by de Melo et al. [28] in the olfactory nerve (their Fig. 5e) do not express olfactory marker protein (OMP), and therefore either are axons of olfactory neurons that have ceased to express OMP, (possibly because they are dying due to the infection [67, 103]), or these axons are not olfactory axons. It is rarely appreciated that some axons in the olfactory nerve are not derived from first-order olfactory neurons that project to the glomeruli in the olfactory bulb but are axons of nervus terminalis neurons that bypass the glomeruli in the olfactory bulb and project to various targets in the forebrain [29, 55, 96], and many of these neurons express ACE2 [9]. The nervus terminalis is a heterogeneous complex of nerve fibers and ganglia that connects the olfactory epithelium with targets in the forebrain caudal to the olfactory bulb [29].
-
7.
Some of the studies localizing SARS-CoV-2 in the brain used antibodies against the spike protein to document virus localization [21, 41, 54, 66, 91]. However, it is now known that the S1 subunit of the spike protein can be shed from the virus during cell entry, and neurons in the brain can take up such cleaved and systemically circulating spike proteins [75, 82]. Accordingly, cells in the brain may contain spike proteins without necessarily containing SARS-CoV-2 virus. When localization of virus RNA was directly compared with localization of spike protein in human autopsy tissues, the large majority of the blood vessels in the brain containing spike protein did not contain any viral RNA [75].
-
8.
Proponents of an olfactory route of SARS-CoV-2 to achieve brain infection often allude to “neuron-hopping” as the mechanism for travel into and within the brain [10, 14, 16, 28, 54, 63, 66, 72, 108]: virus transfer from olfactory receptor neurons to mitral cells (2nd order neurons) in the olfactory bulb, and then transfer to 3rd order neuronal targets in the brain. The time course of virus internalization and subsequent axonal transport by neurons is well established—it takes approximately 24 h for each virus transfer from one neuron to the next-order neuron [4, 33, 73], presumably due to the velocity of kinesin-mediated axonal transport [93]. Previous work established also that neuro-invasive viruses typically infect only structures neuroanatomically linked to the site of inoculation [90]. However, the time course of SARS-CoV-1 and SARS-CoV-2 invasion from the olfactory epithelium to distant targets in the brain, even those that are not 2nd or 3rd order olfactory targets, is much more rapid: the arrival of the virus is approximately simultaneous in the olfactory bulb and in distant brain targets [28, 73, 104], or even “skips” the olfactory bulb [21, 101, 105] or the glomerular layer containing the olfactory axons [104]. These findings do not support the hypothesis that SARS-CoV-2 invades the brain by multiple transfers from neuron to neuron, with the first transfer from olfactory receptor neurons to mitral cells in the olfactory bulb. The observed time course is more consistent with alternative routes of neuro-invasion [5, 39, 81]. Such alternative routes include a pathway that reaches cerebrospinal fluid (CSF)-containing spaces, uses the vasculature, or the virus may travel along a peripheral nerve such as the nervus terminalis that directly innervates the forebrain, including the hypothalamus [96].

Image is adapted from Bryche et al. [15]
SARS-CoV-2 nucleocapsid protein (red) immunolabeled in the olfactory epithelium, double-labeled for olfactory marker protein (OMP, green) and stained with Hoechst nuclear stain (blue). a The SARS-CoV-2 (red) is present in a sustentacular cell that partially overlaps with an OMP-labeled olfactory receptor neuron. b When in the same image the sustentacular cell body is invisible (black ellipsoid shadow with white arrows), as it would be when the plane of section is not entirely perpendicular to the epithelium, then the SARS-CoV-2 protein would be erroneously interpreted to be co-localized within the OMP-expressing olfactory receptor neuron. Scale bar = 10 µm.
Taken together, there are multiple explanations for the seemingly contradictory findings of whether or not olfactory receptor neurons can be infected by SARS-CoV-2 and can carry the virus into the brain. Virus localization within olfactory receptor neurons is ambiguous at best, and there is currently no convincing evidence that the virus travels from the nose to the brain along the axons of olfactory receptor neurons.
When and how does the virus reach the brain?
Brain infection by SARS-CoV-2 has been studied and verified in animal models, primarily in mouse and hamster, in addition to more limited data on humans and non-human primates. Due to species differences of the ACE2 protein [25], SARS-CoV-2 infectivity varies between species. Wild-type mice have low infectivity for SARS-CoV-2, and to study infectivity and virus spread in this species, the SARS-CoV-2 virus has to be mouse-adapted [57, 69], or mice have to be engineered to express human ACE2 instead of, or in addition to, murine ACE2 (Table 1). Multiple such mouse models with different promoters have been developed (reviewed in [19, 43, 69, 76, 80]). Hamsters express an ACE2 protein that results in medium-to-high infectivity of SARS-CoV-2, more similar to the infectivity in humans, and hamsters are therefore deemed to be a more physiological animal model to study neuro-invasion in COVID-19 [24, 25, 28, 103]. In this context, it is important to understand the advantages and limitations of the methods that have been used to provide evidence for the presence of SARS-CoV-2 in tissues, as summarized in Table 4. Plaque formation provides evidence of replicating virus but does not inform about cellular localization. PCR gives evidence of viral RNA but does not inform about virus replication or cellular localization, and it is still uncertain whether subgenomic RNA is indeed an indicator of active replication [1]. In situ hybridization gives evidence of viral RNA and tissue localization. Antibodies against either the spike protein or the nucleocapsid of the virus provide cellular localization but do not distinguish whole virus from cleaved proteins that can circulate systemically in the brain [75]. Evidence at the ultrastructural level is rarely attempted and fraught with uncertainty [47].
In mice that express human ACE2 (hACE2), SARS-CoV-2 infects the olfactory epithelium. Brain infection after intranasal infection seems to depend on the type of promoter used to control the expression of hACE2, as compiled in Table 1 and summarized in Fig. 2. Mouse models that express hACE2 under the control of the endogenous or exogenous murine ACE2 promoter or the cytomegalovirus (CMV) promoter showed mild disease symptoms and only occasionally had SARS-CoV-2 in the brain [2, 45, 80, 89, 98, 105]. In these animals, evidence for virus presence was based mostly on PCR [91, 105], but was not detected by immunocytochemistry or in situ hybridization (Table 1). These “newer” mouse models were generated by CRISPR/cas9 and knock-in approaches, thus the endogenous ACE2 expression is replaced by human ACE2 expression; such mouse models are considered to be more physiologically relevant than the “older” mouse models, although the sometimes-used adenoviral vector may by itself elicit host responses separate from responses to the SARS-CoV-2 infection [43]. The “older” mouse models were generated several years ago for SARS-CoV-1 studies; they have constitutive hACE2 expression controlled by exogenous promoters such as K18 cytokeratin or forkhead box protein J1 (FOXJ1). These mouse models are more often lethal, presumably due to brain infection [21, 41, 50, 54, 76, 80, 89, 101, 104], while the “newer” mouse models and mice infected with mouse-adapted SARS-CoV-2 typically do not show neuro-invasion [57] (Table 1). Other viruses, such as human coronavirus OC43 (HCoV-OC43), mouse hepatitis virus, or herpes simplex virus, readily infect olfactory neurons and move effectively by anterograde axonal transport to secondary and tertiary olfactory centers in the brain [3, 4, 17, 33, 56, 95]. The reason why SARS-CoV-2 rarely infects olfactory circuits in the newer mouse models or in wild-type animals appears to be the lack of expression of the virus entry proteins in olfactory neurons. The viruses that are highly effective neuro-invaders have in common that their entry proteins are abundantly expressed in the neurons that become infected [3, 17, 33, 56, 95].
Probability of brain infection after nasal inoculation in animal models or in SARS-CoV-2 infected patients. Note that the probability of brain infection in humans resembles that in non-transgenic animal models and in the newer human ACE2 (hACE2) mouse models, but not the infection probability in the older transgenic mouse models that use the K18 cytokeratin promoter
In some of the mouse models expressing human ACE2, the timing of arrival of the virus in various brain structures was monitored, and it was described that olfactory bulbs were infected not more than other parts of the brain, especially the hypothalamus and other thalamic nuclei, and the brainstem [21, 101, 104]—similar to some of the human neuropathological findings [66, 86], as described below. The need to engineer human ACE2 expression is a limitation of the mouse animal model, because hACE2 may not be expressed in the same cell types and at the same levels as it is expressed in humans; this may enable neuro-invasion of SARS-CoV-2 that would not occur with normal expression of ACE2. Data from such mice, therefore, need to be interpreted with caution [24]. On the other hand, transgenic mouse models inform about which types of neuro-invasion by SARS-CoV-2 are possible when certain cell types express certain levels of virus entry proteins, and these mice are an important animal model to test mechanisms of neuro-invasion as well as antiviral strategies [21, 104].
In hamsters, seven studies explored if the virus was present in the brain following SARS-CoV-2 nasal inoculation [15, 22, 28, 49, 87, 103]. Four of them found no evidence for brain infection with antibodies (Table 2). Three studies found either viral RNA or virus (by plaque formation) in the olfactory bulb or brain at 1 to 14 days post infection (dpi) [28, 46, 49], with 2 logs lower than in nasal turbinates and with similar levels in the brainstem, cerebral cortex and cerebellum [28]. In cases with positive PCR, virus presence could rarely be confirmed by plaque formation [46], indicating that the large majority of viral RNA was not replicating virus. Using immunohistochemistry or in situ hybridization, five studies reported the absence of viral antigens in the brain or olfactory nerve [15, 49, 87, 102, 103]. One study observed only a few infected (non-neuronal) cells in the olfactory bulb [28]. These discrepancies between studies could be related to the viral titer used during infection. Indeed, the virus titer varies up to 10,000-fold between studies, from 10 plaque-forming units (pfu) [46] to 1 × 105 pfu [103], and the virus was rarely observed in the brain when lower virus titers were used for infection [77]. Similar observations were made in monkeys as described below.
In ferrets, ACE2 has a low virus-binding score, but these animals are susceptible to SARS-CoV-2 infection [25, 35, 84]. As shown in Table 2, SARS-CoV-2 localizes to presumptive sustentacular cells in ferrets [35], similar to hamsters. Only very few animals had virus in the brain clearly above the threshold of detection by PCR [84], and neuro-invasion could not be verified by other methods.
In the physiological animal models (hamster, ferret), the virus was found in the brain only by quantitative PCR or plaque formation, but not by immunocytochemistry (Table 2). This raises the question of the cellular source of the virus. Indeed, if the virus is present only in blood vessels or in circulating immune cells in the brain, virus presence may not be related to neuronal infection. Overall, the studies in hamsters and ferrets do not support brain infection by an olfactory route.
In non-human primates (macaques and African green monkeys), six studies examined SARS-CoV-2 in the brain after nasal or upper respiratory tract inoculation. The first three studies did not find evidence of the virus in the brain using PCR at 3, 4, 7, and 21 days after infection [27, 70, 83] (Table 3). A fourth and fifth study [44, 78] found viral RNA in multiple brain regions at 28 or 35 days post infection, but in one of these studies, the PCR findings could not be verified by antibodies against the nuclear capsid antigen [78]. A sixth study [51] found evidence of virus RNA and nuclear capsid antigen in the brain, including the olfactory bulb, at 1, 4 and 7 days after nasal inoculation. However, this study applied an extremely high dose of virus (107 pfu), about 20 times higher than the other monkey studies (0.7 × 105 and 3 × 105), and more than 100 times the dose of most other animal studies (5 × 103 to 105 pfu). Jiao et al. [51] found viral RNA in the blood and in the CSF already at day 1 after nasal infection. Such a fast appearance of the virus in the CSF essentially precludes neuronal transfer along the olfactory nerve as the sole or primary pathway and instead points to alternative routes of SARS-CoV-2 to achieve brain infection.
In humans, there are no time course studies of neuro-invasion, only reports on the “final outcome.” The virus was found to be abundant in the olfactory epithelium, mostly, if not exclusively, in sustentacular cells [20, 28, 66]. In some patients, the virus was also documented in the brain, with the brainstem, thalamus and hypothalamus more often infected than the olfactory bulb [66]. Virus was also documented in some cases in the cerebral cortex and in the CSF or choroid plexus [16, 26, 34, 63, 65, 66, 71, 75, 86, 89], but it was not detectable in the CSF in other studies [74, 79].
Could the small number of potentially infected olfactory receptor neurons contribute to neuro-invasion of the brain in animals and humans? Most of the reported examples are immature neurons. Immature olfactory receptor neurons cannot transmit the virus to the brain, because they do not have the peripheral and central connections: after the 7–14 days required for the generation of neurons [53], it takes another several day for the immature olfactory neurons to develop cilia [61], and it is thought to take up to 1 week for the immature neurons to grow axons to the appropriate target glomerulus in the olfactory bulb of larger animals or humans [85, 92], although this may occur faster in mice, because of the shorter distances [62].
Taken together, the animal studies examining neuro-invasion via the olfactory nerve and olfactory bulb are inconclusive and rather point to alternative routes. Alternative mechanisms of transfer of SARS-CoV-2 from the nose to the brain include the crossing of the blood–brain-barrier after uptake in leukocytes [5, 14, 77, 108], or entering through the endothelial cells of blood vessels [16, 75, 108], reaching CSF-containing spaces associated with the olfactory nerve [14, 19], or by infecting peripheral processes of nervus terminalis neurons that innervate Bowman’s glands, have free nerve endings in the olfactory epithelium, and innervate blood vessels below the olfactory epithelium [9, 19, 55].
Consequences of brain infection: current controversy
What are the consequences of brain infection with SARS-CoV-2? It is now well established that SARS-CoV-2 can be present—albeit rarely—in the brain of human patients [34, 65, 66, 68, 75, 86, 88], although it needs to be kept in mind that there is an inherent bias because only the most severe (fatal) cases are examined (by autopsy [68, 86]). Not only the route of infection is unclear (as discussed above), but also the consequences of brain infection are currently uncertain, and opinions differ drastically. On the one extreme, it has been proposed that neuro-invasion of the brain may be acutely lethal—animals and patients may die as soon as the brainstem becomes infected, possibly due to shut down of respiratory centers [5, 21, 31, 40, 54, 58, 63, 64, 89]. On the other extreme, it has been noted that brain infection may have little consequence, since there does not seem to be any correlation between the severity of the disease and evidence of the virus in the cerebrospinal fluid (CSF) or brain tissues [65, 74, 86, 88]. An intermediate position is that, as with other viral brain infections, there may be “merely” an increased long-term risk of neurodegenerative diseases due to chronic virus-induced inflammation [30, 32, 39]. It is important to keep in mind that the presence of spike protein or viral RNA does not necessarily mean that the virus actually replicates [1, 82]. It is not yet clear whether the virus or some of the cleaved and circulating viral proteins (spike proteins) typically provoke immune reactions and endothelial cell damage [75] or whether the virus can be present in brains without eliciting any inflammatory or immune reaction (e.g., [89]) or other serious effects such as increased cell death [51]. It is yet uncertain to what extent neurological symptoms in COVID-19 patients are due to a direct viral effect on the brain, or whether neurological symptoms may be primarily due to inflammatory processes, vascular insults, circulating cleaved spike proteins, and other effects of systemic infection in COVID-19 [10, 46, 74,75,76, 78, 79, 86, 88, 108]. Without a doubt, new insights into these controversies will emerge as the pandemic continues.
Conclusions
We question an olfactory neuron route of SARS-CoV-2 to the brain for multiple reasons:
-
There is a wide consensus that the large majority of mature olfactory receptor neurons do not express the obligatory virus entry proteins.
-
Many reports of the virus within olfactory receptor neurons neglect the fact that sustentacular cells tightly wrap these neurons, making it possible to observe false positives even when cell-type-specific markers are used.
-
The few infected olfactory receptor neurons reported in some studies are mostly immature cells, but they lack axonal projections to transport the virus into the brain.
-
The timeline of neuro-invasion in animal models indicates that the virus uses alternative routes rather than neuron-hopping and virus transfer between olfactory neurons.
-
Neuro-invasion of SARS-CoV-2 has consistently been described for a non-physiological mouse model (with transgenic expression of human ACE2 via the K18 promoter), but reports of such neuro-invasion are rare in physiological animal models using the endogenous ACE2 promoter.
Taken together, the current evidence from animal models and human tissues supports the notion that the lack of entry protein expression in olfactory neurons creates a formidable barrier that makes it unlikely for SARS-CoV-2 to gain access to the olfactory bulb along the olfactory nerve axons. It should be noted that this does not rule out a pathway from the nose to the brain by other mechanisms: a vascular route [5, 14, 16, 75, 77, 108], a route through CSF spaces [14, 19], and a route along with the nervus terminalis system [9, 19] or the Grueneberg ganglion [12]. The current evidence favors alternative routes from the nose to the brain, at least in the acute phase (first two weeks) of infection. Since the viral load typically reduces rapidly within the first week of infection [94], the brain appears to be protected in the vast majority of cases with SARS-CoV-2 infection. We are concerned that studies advocating an olfactory route for SARS-CoV-2 to infect the brain may unnecessarily alarm a large number of patients suffering from anosmia. The COVID-19 pandemic is intimidating; our critical review of the evidence indicates that, contrary to several attention-grabbing publications, infection of the olfactory epithelium causing loss of smell in COVID-19 is rarely followed by a brain infection.
References
Alexandersen S, Chamings A, Bhatta TR (2020) SARS-CoV-2 genomic and subgenomic RNAs in diagnostic samples are not an indicator of active replication. Nat Commun 11(1):6059. https://doi.org/10.1038/s41467-020-19883-7
Bao L, Deng W, Huang B, Gao H, Liu J, Ren L et al (2020) The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature 583:830–833. https://doi.org/10.1038/s41586-020-2312-y
Barnett EM, Cassell MD, Perlman S (1993) Two neurotropic viruses, herpes simplex virus type 1 and mouse hepatitis virus, spread along different neural pathways from the main olfactory bulb. Neuroscience 57:1007–1025. https://doi.org/10.1016/0306-4522(93)90045-h
Barnett EM, Evans GD, Sun N, Perlman S, Cassell MD (1995) Anterograde tracing of trigeminal afferent pathways from the murine tooth pulp to cortex using herpes simplex virus type 1. J Neurosci 15:2972–2984. https://doi.org/10.1523/JNEUROSCI.15-04-02972.1995
Barrantes FJ (2020) Central nervous system targets and routes for SARS-CoV-2: current views and new hypotheses. ACS Chem Neurosci 11:2793–2803. https://doi.org/10.1021/acschemneuro.0c00434
Baxter BD, Larson ED, Feinstein P, Polese AG, Bubak AN, Niemeyer CS et al (2020) Transcriptional profiling reveals TRPM5-expressing cells involved in viral infection in the olfactory epithelium. bioRxiv. https://doi.org/10.1101/2020.05.14.096016 (Preprint)
Bilinska K, Butowt R (2020) Anosmia in COVID-19: a bumpy road to establishing a cellular mechanism. ACS Chem Neurosci 11:2152–2155. https://doi.org/10.1021/acschemneuro.0c00406
Bilinska K, Jakubowska P, Von Bartheld CS, Butowt R (2020) Expression of the SARS-CoV-2 entry proteins, ACE2 and TMPRSS2, in cells of the olfactory epithelium: identification of cell types and trends with age. ACS Chem Neurosci 11:1555–1562. https://doi.org/10.1021/acschemneuro.0c00210
Bilinska K, von Bartheld CS, Butowt R (2021) Expression of the ACE2 virus entry protein in the nervus terminalis suggests an alternative route for brain infection in COVID-19. bioRxiv. https://doi.org/10.1101/2021.04.11.439398 (Preprint)
Bougakov D, Podell K, Goldberg E (2021) Multiple neuroinvasive pathways in COVID-19. Mol Neurobiol 58:564–575. https://doi.org/10.1007/s12035-020-02152-5
Brann DH, Tsukahara T, Weinreb C, Lipovsek M, Van den Berge K, Gong B et al (2020) Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci Adv 6(31):eabc5801. https://doi.org/10.1126/sciadv.abc5801
Brechbühl J, Wood D, Bouteiller S, Lopes AC, Verdumo C, Broillet M-C (2021) Age-dependent appearance of SARS-CoV-2 entry cells in mouse chemosensory systems reflects COVID-19 anosmia and ageusia symptoms. bioRxiv. https://doi.org/10.1101/2021.03.29.437530 (Preprint)
Breipohl W, Laugwitz HJ, Bornfeld N (1974) Topological relations between the dendrites of olfactory sensory cells and sustentacular cells in different vertebrates. An ultrastructural study. J Anat 117:89–94
Briguglio M, Bona A, Porta M, Dell’Osso B, Pregliasco FE, Banfi G (2020) Disentangling the hypothesis of host dysosmia and SARS-CoV-2: the bait symptom that hides neglected neurophysiological routes. Front Physiol 11:671. https://doi.org/10.3389/fphys.2020.00671
Bryche B, St Albin A, Murri S, Lacôte S, Pulido C, Ar Gouilh M et al (2020) Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. Brain Behav Immun 89:579–586. https://doi.org/10.1016/j.bbi.2020.06.032
Burks SM, Rosas-Hernandez H, Alenjandro Ramirez-Lee M, Cuevas E, Talpos JC (2021) Can SARS-CoV-2 infect the central nervous system via the olfactory bulb or the blood-brain barrier? Brain Behav Immun. https://doi.org/10.1016/j.bbi.2020.12.031
Butler N, Pewe L, Trandem K, Perlman S (2006) Murine encephalitis caused by HCoV-OC43, a human coronavirus with broad species specificity, is partly immune-mediated. Virology 347:410–421. https://doi.org/10.1016/j.virol.2005.11.044
Butowt R, Bilinska K (2020) SARS-CoV-2: olfaction, brain infection, and the urgent need for clinical samples allowing earlier virus detection. ACS Chem Neurosci 11:1200–1203. https://doi.org/10.1021/acschemneuro.0c00172
Butowt R, von Bartheld CS (2020) Anosmia in COVID-19: underlying mechanisms and assessment of an olfactory route to brain infection. Neuroscientist. https://doi.org/10.1177/1073858420956905
Cantuti-Castelvetri L, Ojha R, Pedro LD, Djannatian M, Franz J, Kuivanen S et al (2020) Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 370:856–860. https://doi.org/10.1126/science.abd2985
Carossino M, Montanaro P, O’Connell A, Kenney D, Gertje H, Grosz KA et al (2021) Fatal neuroinvasion of SARS-CoV-2 in K18-hACE2 mice is partially dependent on hACE2 expression. bioRxiv. https://doi.org/10.1101/2021.01.13.425144 (Preprint)
Chan JF, Zhang AJ, Yuan S, Poon VK, Chan CC, Lee AC et al (2020) Simulation of the clinical and pathological manifestations of coronavirus disease 2019 (COVID-19) in a golden syrian hamster model: implications for disease pathogenesis and transmissibility. Clin Infect Dis 71:2428–2446. https://doi.org/10.1093/cid/ciaa325
Chen M, Shen W, Rowan NR, Kulaga H, Hillel A, Ramanathan M Jr et al (2020) Elevated ACE-2 expression in the olfactory neuroepithelium: implications for anosmia and upper respiratory SARS-CoV-2 entry and replication. Eur Respir J 56:2001948. https://doi.org/10.1183/13993003.01948-2020
Cooper KW, Brann DH, Farruggia MC, Bhutani S, Pellegrino R, Tsukahara T et al (2020) COVID-19 and the chemical senses: supporting players take center stage. Neuron 107:219–233. https://doi.org/10.1016/j.neuron.2020.06.032
Damas J, Hughes GM, Keough KC, Painter CA, Persky NS, Corbo M et al (2020) Broad host range of SARS-CoV-2 predicted by comparative and structural analysis of ACE2 in vertebrates. Proc Natl Acad Sci USA 117:22311–22322. https://doi.org/10.1073/pnas.2010146117
Deffner F, Scharr M, Klingenstein S, Klingenstein M, Milazzo A, Scherer S et al (2020) Histological evidence for the enteric nervous system and the choroid plexus as alternative routes of neuroinvasion by SARS-CoV2. Front Neuroanat 14:596439. https://doi.org/10.3389/fnana.2020.596439
Deng W, Bao L, Gao H, Xiang Z, Qu Y, Song Z et al (2020) Ocular conjunctival inoculation of SARS-CoV-2 can cause mild COVID-19 in rhesus macaques. Nat Commun 11:4400. https://doi.org/10.1038/s41467-020-18149-6
de Melo GD, Lazarini F, Levallois S, Hautefort C, Michel V, Larrous F et al (2020) COVID-19 associated olfactory dysfunction reveals SARS-CoV-2 neuroinvasion and persistence in the olfactory system. bioRxiv. https://doi.org/10.1101/2020.11.18.388819 (Preprint)
Demski LS (1993) Terminal nerve complex. Acta Anat (Basel) 148:81–95. https://doi.org/10.1159/000147528
Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L, Dubé M et al (2019) Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses 12(1):14. https://doi.org/10.3390/v12010014
Dey J, Alam MT, Chandra S, Gupta J, Ray U, Srivastava AK et al (2021) Neuroinvasion of SARS-CoV-2 may play a role in the breakdown of respiratory center of the brain. J Med Virol 93:1296–1303. https://doi.org/10.1002/jmv.26521
Doty RL (2008) The olfactory vector hypothesis of neurodegenerative disease: is it viable? Ann Neurol 63:7–15. https://doi.org/10.1002/ana.21327
Dubé M, Le Coupanec A, Wong AHM, Rini JM, Desforges M, Talbot PJ (2018) Axonal transport enables neuron-to-neuron propagation of human coronavirus OC43. J Virol 92(17):e00404-e418. https://doi.org/10.1128/JVI.00404-18
Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A et al (2020) Neurological associations of COVID-19. Lancet Neurol 19:767–783. https://doi.org/10.1016/S1474-4422(20)30221-0
Everett HE, Lean FZX, Byrne AMP, van Diemen PM, Rhodes S, James J et al (2021) Intranasal infection of ferrets with SARS-CoV-2 as a model for asymptomatic human infection. Viruses 13:113. https://doi.org/10.3390/v13010113
Falk N, Lösl M, Schröder N, Gießl A (2015) Specialized cilia in mammalian sensory systems. Cells 4:500–519. https://doi.org/10.3390/cells4030500
Finger TE, Bartel DL, Shultz N, Goodson NB, Greer CA (2017) 5HTR3A-driven GFP labels immature olfactory sensory neurons. J Comp Neurol 525:1743–1755. https://doi.org/10.1002/cne.24180
Fodoulian L, Tuberosa J, Rossier D, Boillat M, Kan C, Pauli V et al (2020) SARS-CoV-2 receptors and entry genes are expressed in the human olfactory neuroepithelium and brain. iScience 23(12):101839. https://doi.org/10.1016/j.isci.2020.101839
Forrester JV, McMenamin PG, Dando SJ (2018) CNS infection and immune privilege. Nat Rev Neurosci 19:655–671. https://doi.org/10.1038/s41583-018-0070-8
Gandhi S, Srivastava AK, Ray U, Tripathi PP (2020) Is the collapse of the respiratory center in the brain responsible for respiratory breakdown in COVID-19 patients? ACS Chem Neurosci 11:1379–1381. https://doi.org/10.1021/acschemneuro.0c00217
Golden JW, Cline CR, Zeng X, Garrison AR, Carey BD, Mucker EM et al (2020) Human angiotensin-converting enzyme 2 transgenic mice infected with SARS-CoV-2 develop severe and fatal respiratory disease. JCI Insight 5(19):e142032. https://doi.org/10.1172/jci.insight.142032
Gupta K, Mohanty SK, Mittal A, Kalra S, Kumar S, Mishra T et al (2021) The cellular basis of the loss of smell in 2019-nCoV-infected individuals. Brief Bioinform 22:873–881. https://doi.org/10.1093/bib/bbaa168
Harker JA, Johansson C (2021) Rapidly deployable mouse models of SARS-CoV-2 infection add flexibility to the COVID-19 toolbox. Am J Respir Cell Mol Biol 64:7–9. https://doi.org/10.1165/rcmb.2020-0456ED
Hartman AL, Nambulli S, McMillen CM, White AG, Tilston-Lunel NL, Albe JR et al (2020) SARS-CoV-2 infection of African green monkeys results in mild respiratory disease discernible by PET/CT imaging and shedding of infectious virus from both respiratory and gastrointestinal tracts. PLoS Pathog 16(9):e1008903. https://doi.org/10.1371/journal.ppat.1008903
Hassan AO, Case JB, Winkler ES, Thackray LB, Kafai NM, Bailey AL et al (2020) A SARS-CoV-2 infection model in mice demonstrates protection by neutralizing antibodies. Cell 182:744-753.e4. https://doi.org/10.1016/j.cell.2020.06.011
Hoagland DA, Møller R, Uhl SA, Oishi K, Frere K, Golynker I et al (2021) Leveraging the antiviral type I interferon system as a first line of defense against SARS-CoV-2 pathogenicity. Immunity 54:557-570.e5. https://doi.org/10.1016/j.immuni.2021.01.017
Hopfer H, Herzig MC, Gosert R, Menter T, Hench J, Tzankov A et al (2021) Hunting coronavirus by transmission electron microscopy—a guide to SARS-CoV-2-associated ultrastructural pathology in COVID-19 tissues. Histopathology 78:358–370. https://doi.org/10.1111/his.14264
Hou YJ, Okuda K, Edwards CE, Martinez DR, Asakura T, Dinnon KH 3rd et al (2020) SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell 182:429-446.e14. https://doi.org/10.1016/j.cell.2020.05.042
Imai M, Iwatsuki-Horimoto K, Hatta M, Loeber S, Halfmann PJ, Nakajima N et al (2020) Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc Natl Acad Sci USA 117:16587–16595. https://doi.org/10.1073/pnas.2009799117
Jiang RD, Liu MQ, Chen Y, Shan C, Zhou YW, Shen XR et al (2020) Pathogenesis of SARS-CoV-2 in transgenic mice expressing human angiotensin-converting enzyme 2. Cell 182:50-58.e8. https://doi.org/10.1016/j.cell.2020.05.027
Jiao L, Yang Y, Yu W, Zhao Y, Long H, Gao J et al (2020) SARS-CoV-2 invades the central nervous system via the olfactory route in rhesus monkeys. SSRN. https://doi.org/10.2139/ssrn.3689615 (Preprint)
Klingenstein M, Klingenstein S, Neckel PH, Mack AF, Wagner AP, Kleger A et al (2021) Evidence of SARS-CoV2 entry protein ACE2 in the human nose and olfactory bulb. Cells Tissues Organs 22:1–10. https://doi.org/10.1159/000513040
Kondo K, Suzukawa K, Sakamoto T, Watanabe K, Kanaya K, Ushio M et al (2010) Age-related changes in cell dynamics of the postnatal mouse olfactory neuroepithelium: cell proliferation, neuronal differentiation, and cell death. J Comp Neurol 518:1962–1975. https://doi.org/10.1002/cne.22316
Kumari P, Rothan HA, Natekar JP, Stone S, Pathak H, Strate PG et al (2021) Neuroinvasion and encephalitis following intranasal inoculation of SARS-CoV-2 in K18-hACE2 mice. Viruses 13(1):132. https://doi.org/10.3390/v13010132
Larsell O (1950) The nervus terminalis. Ann Otol Rhinol Laryngol 59:414–438. https://doi.org/10.1177/000348945005900211
Le Coupanec A, Desforges M, Kaufer B, Dubeau P, Côté M, Talbot PJ (2021) Potential differences in cleavage of the S protein and type-1 interferon together control human coronavirus infection, propagation, and neuropathology within the central nervous system. J Virol (Epub). https://doi.org/10.1128/JVI.00140-21
Leist SR, Dinnon KH 3rd, Schäfer A, Tse LV, Okuda K, Hou YJ et al (2020) A mouse-adapted SARS-CoV-2 induces acute lung injury and mortality in standard laboratory mice. Cell 183:1070-1085.e12. https://doi.org/10.1016/j.cell.2020.09.050
Li YC, Bai WZ, Hashikawa T (2020) The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol 92:552–555. https://doi.org/10.1002/jmv.25728
Li Z, Liu T, Yang N, Han D, Mi X, Li Y et al (2020) Neurological manifestations of patients with COVID-19: potential routes of SARS-CoV-2 neuroinvasion from the periphery to the brain. Front Med 14:533–541. https://doi.org/10.1007/s11684-020-0786-5
Liang F (2018) Olfactory receptor neuronal dendrites become mostly intra-sustentacularly enwrapped upon maturity. J Anat 232:674–685. https://doi.org/10.1111/joa.12777
Liang F (2020) Sustentacular cell enwrapment of olfactory receptor neuronal dendrites: an update. Genes (Basel) 11(5):493. https://doi.org/10.3390/genes11050493
Liberia T, Martin-Lopez E, Meller SJ, Greer CA (2019) Sequential maturation of olfactory sensory neurons in the mature olfactory epithelium. eNeuro 6(5). https://doi.org/10.1523/ENEURO.0266-19.2019
Liu JM, Tan BH, Wu S, Gui Y, Suo JL, Li YC (2021) Evidence of central nervous system infection and neuroinvasive routes, as well as neurological involvement, in the lethality of SARS-CoV-2 infection. J Med Virol 93:1304–1313. https://doi.org/10.1002/jmv.26570
Machado C, DeFina PA, Chinchilla M, Machado Y, Machado Y (2020) Brainstem dysfunction in SARS-COV-2 infection can be a potential cause of respiratory distress. Neurol India 68:989–993. https://doi.org/10.4103/0028-3886.299165
Matschke J, Lütgehetmann M, Hagel C, Sperhake JP, Schröder AS, Edler C et al (2020) Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol 19:919–929. https://doi.org/10.1016/S1474-4422(20)30308-2
Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R et al (2021) Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci 24:168–175. https://doi.org/10.1038/s41593-020-00758-5
Mori I, Goshima F, Imai Y, Kohsaka S, Sugiyama T, Yoshida T et al (2002) Olfactory receptor neurons prevent dissemination of neurovirulent influenza A virus into the brain by undergoing virus-induced apoptosis. J Gen Virol 83:2109–2116. https://doi.org/10.1099/0022-1317-83-9-2109
Mukerji SS, Solomon IH (2021) What can we learn from brain autopsy in COVID-19? Neurosci Lett 742:135528. https://doi.org/10.1016/j.neulet.2020.135528
Muñoz-Fontela C, Dowling WE, Funnell SGP, Gsell PS, Riveros-Balta AX, Albrecht RA et al (2020) Animal models for COVID-19. Nature 586:509–515. https://doi.org/10.1038/s41586-020-2787-6
Munster VJ, Feldmann F, Williamson BN, van Doremalen N, Pérez-Pérez L, Schulz J et al (2020) Respiratory disease in rhesus macaques inoculated with SARS-CoV-2. Nature 585:268–272. https://doi.org/10.1038/s41586-020-2324-7
Nampoothiri S, Sauve S, Ternier G, Fernandois D, Coelho C, Imbernon M et al (2020) The hypothalamus as a hub for putative SARS-CoV-2 brain infection. bioRxiv. https://doi.org/10.1101/2020.06.08.139329 (Preprint)
Natoli S, Oliveira V, Calabresi P, Maia LF, Pisani A (2020) Does SARS-Cov-2 invade the brain? Translational lessons from animal models. Eur J Neurol 27:1764–1773. https://doi.org/10.1111/ene.14277
Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S (2008) Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol 82:7264–7275. https://doi.org/10.1128/JVI.00737-0
Neumann B, Schmidbauer ML, Dimitriadis K, Otto S, Knier B, Niesen WD et al (2020) Cerebrospinal fluid findings in COVID-19 patients with neurological symptoms. J Neurol Sci 418:117090. https://doi.org/10.1016/j.jns.2020.117090
Nuovo GJ, Magro C, Shaffer T, Awad H, Suster D, Mikhail S et al (2021) Endothelial cell damage is the central part of COVID-19 and a mouse model induced by injection of the S1 subunit of the spike protein. Ann Diagn Pathol 51:151682. https://doi.org/10.1016/j.anndiagpath.2020.151682
Oladunni FS, Park JG, Pino PA, Gonzalez O, Akhter A, Allué-Guardia A et al (2020) Lethality of SARS-CoV-2 infection in K18 human angiotensin-converting enzyme 2 transgenic mice. Nat Commun 11(1):6122. https://doi.org/10.1038/s41467-020-19891-7
Pellegrini L, Albecka A, Mallery DL, Kellner MJ, Paul D, Carter AP et al (2020) SARS-CoV-2 infects the brain choroid plexus and disrupts the blood-CSF barrier in human brain organoids. Cell Stem Cell 27:951-961.e5. https://doi.org/10.1016/j.stem.2020.10.001
Philippens IHCHM, Böszörményi KP, Wubben JA, Fagrouch ZC, van Driel N, Mayenburg AQ et al (2021) SARS-CoV-2 causes brain inflammation and induces Lewy body formation in macaques. bioRxiv. https://doi.org/10.1101/2021.02.23.432474 (Preprint)
Placantonakis DG, Aguero-Rosenfeld M, Flaifel A, Colavito J, Inglima K, Zagzag D et al (2020) SARS-CoV-2 is not detected in the cerebrospinal fluid of encephalopathic COVID-19 patients. Front Neurol 11:587384. https://doi.org/10.3389/fneur.2020.587384
Rathnasinghe R, Strohmeier S, Amanat F, Gillespie VL, Krammer F, García-Sastre A et al (2020) Comparison of transgenic and adenovirus hACE2 mouse models for SARS-CoV-2 infection. Emerg Microbes Infect 9:2433–2445. https://doi.org/10.1080/22221751.2020.1838955
Reza-Zaldívar EE, Hernández-Sapiéns MA, Minjarez B, Gómez-Pinedo U, Márquez-Aguirre AL, Mateos-Díaz JC et al (2021) Infection mechanism of SARS-COV-2 and its implication on the nervous system. Front Immunol 11:621735. https://doi.org/10.3389/fimmu.2020.621735
Rhea EM, Logsdon AF, Hansen KM, Williams LM, Reed MJ, Baumann KK et al (2021) The S1 protein of SARS-CoV-2 crosses the blood-brain barrier in mice. Nat Neurosci 24:368–378. https://doi.org/10.1038/s41593-020-00771-8
Rockx B, Kuiken T, Herfst S, Bestebroer T, Lamers MM, Oude Munnink BB et al (2020) Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science 368:1012–1015. https://doi.org/10.1126/science.abb7314
Schlottau K, Rissmann M, Graaf A, Schön J, Sehl J, Wylezich C et al (2020) SARS-CoV-2 in fruit bats, ferrets, pigs, and chickens: an experimental transmission study. Lancet Microbe 1(5):e218–e225. https://doi.org/10.1016/S2666-5247(20)30089-6
Schwob JE (2002) Neural regeneration and the peripheral olfactory system. Anat Rec 269:33–49. https://doi.org/10.1002/ar.10047
Serrano GE, Walker JE, Arce R, Glass MJ, Vargas D, Sue LI et al (2021) Mapping of SARS-CoV-2 brain invasion and histopathology in COVID-19 disease. medRxiv. https://doi.org/10.1101/2021.02.15.21251511 (Preprint)
Sia SF, Yan LM, Chin AWH, Fung K, Choy KT, Wong AYL et al (2020) Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature 583:834–838. https://doi.org/10.1038/s41586-020-2342-5
Solomon T (2021) Neurological infection with SARS-CoV-2—the story so far. Nat Rev Neurol 17:65–66. https://doi.org/10.1038/s41582-020-00453-w
Song E, Zhang C, Israelow B, Lu-Culligan A, Prado AV, Skriabine S et al (2021) Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exp Med 218(3):e20202135. https://doi.org/10.1084/jem.20202135
Sun N, Cassell MD, Perlman S (1996) Anterograde, transneuronal transport of herpes simplex virus type 1 strain H129 in the murine visual system. J Virol 70:5405–5413. https://doi.org/10.1128/JVI.70.8.5405-5413.1996
Sun SH, Chen Q, Gu HJ, Yang G, Wang YX, Huang XY et al (2020) A mouse model of SARS-CoV-2 infection and pathogenesis. Cell Host Microbe 28:124-133.e4. https://doi.org/10.1016/j.chom.2020.05.020
Suzuki Y, Takeda M, Farbman AI (1996) Supporting cells as phagocytes in the olfactory epithelium after bulbectomy. J Comp Neurol 376:509–517. https://doi.org/10.1002/(SICI)1096-9861(19961223)376:4%3c509::AID-CNE1%3e3.0.CO;2-5
Taylor MP, Enquist LW (2015) Axonal spread of neuroinvasive viral infections. Trends Microbiol 23:283–288. https://doi.org/10.1016/j.tim.2015.01.002
Urata S, Maruyama J, Kishimoto-Urata M, Sattler RA, Cook R, Lin N et al (2021) Regeneration profiles of olfactory epithelium after SARS-CoV-2 infection in golden syrian hamsters. ACS Chem Neurosci 12:589–595. https://doi.org/10.1021/acschemneuro.0c00649
van Riel D, Verdijk R, Kuiken T (2015) The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. J Pathol 235:277–287. https://doi.org/10.1002/path.4461
von Bartheld CS (2004) The terminal nerve and its relation with extrabulbar “olfactory” projections: lessons from lampreys and lungfishes. Microsc Res Tech 65:13–24. https://doi.org/10.1002/jemt.20095
von Bartheld CS, Hagen MM, Butowt R (2020) Prevalence of chemosensory dysfunction in COVID-19 patients: a systematic review and meta-analysis reveals significant ethnic differences. ACS Chem Neurosci 11:2944–2961. https://doi.org/10.1021/acschemneuro.0c00460
Ye Q, Zhou J, Yang G, Li R-T, He Q, Zhang Y et al (2020) SARS-CoV-2 infection causes transient olfactory dysfunction in mice. bioRxiv. https://doi.org/10.1101/2020.11.10.376673 (Preprint)
Yinda CK, Port JR, Bushmaker T, Offei Owusu I, Purushotham JN, Avanzato VA et al (2021) K18-hACE2 mice develop respiratory disease resembling severe COVID-19. PLoS Pathog 17(1):e1009195. https://doi.org/10.1371/journal.ppat.1009195
Wang W, Xu Y, Gao R, Lu R, Han K, Wu G et al (2020) Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 323:1843–1844. https://doi.org/10.1001/jama.2020.3786
Winkler ES, Bailey AL, Kafai NM, Nair S, McCune BT, Yu J et al (2020) SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat Immunol 21:1327–1335. https://doi.org/10.1038/s41590-020-0778-2
Zazhytska M, Kodra A, Hoagland DA, Fullard JF, Shayya H, Omer A et al (2021) Disruption of nuclear architecture as a cause of COVID-19 induced anosmia. bioRxiv. https://doi.org/10.1101/2021.02.09.430314 (Preprint)
Zhang AJ, Lee AC, Chu H, Chan JF, Fan Z, Li C et al (2020) SARS-CoV-2 infects and damages the mature and immature olfactory sensory neurons of hamsters. Clin Infect Dis 15:ciaa995. https://doi.org/10.1093/cid/ciaa995
Zheng J, Wong LR, Li K, Verma AK, Ortiz M, Wohlford-Lenane C et al (2021) COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice. Nature 589:603–607. https://doi.org/10.1038/s41586-020-2943-z
Zhou B, Thao TTN, Hoffmann D, Taddeo A, Ebert N, Labroussaa F et al (2020) SARS-CoV-2 spike D614G variant confers enhanced replication and transmissibility. bioRxiv. https://doi.org/10.1101/2020.10.27.357558 (Preprint)
Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN et al (2020) SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 181:1016-1035.e19. https://doi.org/10.1016/j.cell.2020.04.035
Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z et al (2020) SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med 382:1177–1179. https://doi.org/10.1056/NEJMc2001737
Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S (2020) Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol 77:1018–1027. https://doi.org/10.1001/jamaneurol.2020.2065
Acknowledgements
The authors thank Katarzyna Bilinska (Nicolaus Copernicus University, Poland), Sandeep R. Datta (Harvard Medical School), and Dennis Mathew (University of Nevada, Reno) for helpful comments and discussions. Our work was supported by the “Excellence Initiative-Research University" programme at the Nicolaus Copernicus University (R.B.), a grant from the SA INRAE Department (N.M.), and grant GM103554 from the National Institutes of Health (C.S.v.B.).
Author information
Authors and Affiliations
Contributions
CSvB, RB, and NM designed the study, all authors contributed to the writing and approved the final version.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Butowt, R., Meunier, N., Bryche, B. et al. The olfactory nerve is not a likely route to brain infection in COVID-19: a critical review of data from humans and animal models. Acta Neuropathol 141, 809–822 (2021). https://doi.org/10.1007/s00401-021-02314-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-021-02314-2