Abstract
Modelling capabilities have drastically improved in the last decade. However, in most of the cases the fire response of building elements is predicted by fitting input material properties to the models in order to match test data. This paper presents models developed to predict the unexposed side temperature of stone wool layered composites with stainless steel or gypsum claddings exposed to severe heat conditions. The suitability of material thermal properties from literature and reaction kinetic parameters obtained at bench scale (e.g. thermogravimetric analysis, bomb calorimeter, slug test) to model composites at different heat exposures is studied. Modelling efforts include: (1) the combustion of the organic content of the wool, (2) diffusion term to account for the passage of hot air through the wool, (3) calcination reactions in the gypsum plasterboard, (4) energy released by burning of the paper lining of gypsum plasterboard. The models are compared against experimental data. Results show that material thermal properties of gypsum plasterboard and stone wool retrieved from the literature and obtained at a bench scale provide accurate model predictions under different heat exposures. Furthermore, reactions schemes for the dehydration of gypsum plasterboard and organic content combustion in the wool also provide good modelling results. Further analysis is necessary to understand the environmental conditions inside the layered composites in fire exposures in order to achieve better modelling predictions.
Similar content being viewed by others
1 Introduction
The adequate fire performance of building elements is traditionally assessed according to standardized resistance to fire tests. In these tests a construction is deemed to be safe if well-defined insulation, stability and integrity criteria are fulfilled for the rating time, under a standardized temperature time exposure [1, 2]. However, standard testing presents certain limitations. For instance, it is not feasible to test all possible constructions [3], thus in many cases an expert judgment is provided without actual testing of all certified configurations. Moreover, standard fire testing provides limited information about the performance of the construction in fire conditions representing real compartment fires. In the last decades, together with the advancement of computational capabilities, models have been developed to predict the behaviour of building elements in fire. Accurate model predictions can help manufacturers during their product development process, before performing expensive tests, and can as well provide fire safety engineers with necessary tools to design buildings based on the performance under realistic fire conditions.
Modelling material response to fire exposure is extremely complex. When exposed to heat, building materials undergo a series of degradation and decomposition processes, such as melting, cracking, burning, exothermic and endothermic chemical reactions, etc., that hinder their modelling. In addition, parameters defining boundary conditions, such as convective heat transfer coefficients, emissivity and absorptivity, and thermal properties of materials at elevated temperatures are in many cases uncertain and difficult to define [4, 5]. Thus, it has been a common approach to model by fitting input material properties to standardized fire resistance tests results (e.g. gypsum building barriers [6,7,8]). In many cases, these models fail to predict other fire scenarios than the ones they have been fitted for [9, 10]. An alternative approach to fitting input material properties is to obtain them through smaller scale testing [11,12,13,14]. This includes bench scale tests to obtain thermal conductivity (e.g. slug calorimeter, transient plane source, heat flow meter) and micro-scale testing to study reaction kinetics and thermochemistry (e.g. thermogravimetric analysis TGA, micro-combustion calorimetry MCC, differential scanning calorimetry DSC, bomb calorimetry). This approach can be refered to as multi-scale or scaling-up approach, and has been used in numerous occasion in different aspects of fire modelling [15,16,17,18,19]. Yet, the use of material thermal properties and reaction kinetics obtained at bench scales to model larger scales is not straightforward. Materials thermal response is sensitive to changes in the micro-structure. Thus, materials thermal properties might vary depending on testing parameters such as the heating rate [20], or reactions observed at smaller scale might occur differently at larger scales depending on environmental conditions such as availability of oxygen to undergo combustion. Thus, increasing the complexity of the modelling would not always lead to better model predictions [21, 22]. Appropriate levels of complexity should be used when modelling the heat transfer response of materials to fire.
This paper investigates the use of a multi-scale approach for modelling stone wool composites based on material properties obtained through small scale tests. The methodology followed consists on sequentially increasing the complexity of the model and compare it to experimental data. Heat transfer modelling is used to predict the behaviour of stone wool insulated layered composites with steel or gypsum plasterboard claddings exposed to four different heating regimes: (1) a constant incident heat flux of 7 kW/m2, (2) a constant incident heat flux of 60 kW/m2, (3) a variable incident heat flux ranging up to 62 kW/m2 and (4) an ISO 834 temperature time exposure. Material thermal properties obtained at bench scale and reaction kinetic parameters are retrieved from literature. Modelling efforts include: heat conduction through the composites, heat and mass transfer through the composite accounting for pressure driven movement of hot air through the stone wool, combustion of organic content of stone wool, dehydration of gypsum plasterboard, and burning of the paper lining of gypsum plasterboard. The developed models are based on the small scale analysis on stone wool from Livkiss et al. [23] and experimental data from Andres et al. [24]. The models are limited to one-dimensional analysis, disregarding the effect of studding in the composites.
2 Constructions and Overview of the Models
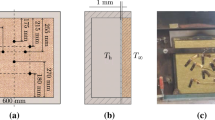
One-dimensional heat transfer analyses have been performed to predict the thermal evolution of stone wool layered constructions under different heat exposures. Table 1 shows the composite’s dimensions and Tables 2 and 3 the summary of the modelling work involved. The models are built based on the experimental data presented in Andres et al. [24]. The modelling approach followed consists on sequentially increasing the complexity of the composite and the model. The first composite modelled is steel- stone wool- steel composite (S-SW-S), where the only expected reaction occurring in the materials involved is the thermal decomposition and combustion of the organic content in the wool. Thus, heat transfer model with and without sub-models accounting for the reaction are developed. The second composite involved in the study is stone wool-steel (SW-S) composite; in this case, the stone wool is directly exposed to the heat as there is no steel cladding on the exposed side. Thus, hot air coming from the exposed side can penetrate the porous media of the stone wool. In order to account for the passage of hot air a model including mass transfer is considered. The third composite modelled is gypsum-stone wool-gypsum composite (G-SW-G). In these constructions reactions due to the calcination of the board and burning of the paper lining are also expected.
3 Models and Material Sub-Models
3.1 Heat Transfer Model
The transient one-dimensional heat transfer model is implemented in COMSOL Multiphysics®[25]. It consists on heat conduction through the materials (Eq. 1). Materials are regarded as solids, and therefore there is no consideration of porosity and convective heat transfer through the porous media.
Figure 1 shows a schematic representation of the boundary conditions for the heat transfer, where the exposed side is referred to as ‘hot’, whereas the unexposed side as ‘cold’. From the exposures modelled there are two very different scenarios, on one hand there are furnace tests where samples are exposed to ISO 834 [1] temperature–time curve, on the other hand radiant panel tests where samples are in an open space and exposed to a constant or variable incident heat exposures. In each case the boundaries are represented differently. In furnace tests the convective and radiative boundary conditions (Eqs. 2, 3) on the exposed side to the fire and the unexposed side are:
In Eqs. 2, 3 the emissivities and absorptivities of the materials are assumed to be equal following Kirchoff law. The exposure temperature (Tg) is assumed to be the incident black body radiation temperature from the furnace exposure [26]. T is the specimen surface temperature, Tamb is the ambient temperature on the unexposed side of the specimen, and \(\dot{q}^{\prime \prime }\) represents the net heat flux to the specimen.
For samples exposed to an incident heat flux from a radiant panel (H-TRIS [27]), the unexposed boundary condition follows Eq. 3 and the exposed boundary condition is applied as an incident heat flux set from the radiant panels and thermal losses due to the radiation and convection to the environment as shown in Eq. 4.
where \(\dot{q}_{in}^{^{\prime\prime}}\) is the effective incident heat flux at the surface of the specimen. The boundary parameters used for modelling are shown in Table 4. Specimens tested with H-TRIS were coated on the exposed side with a solar collector paint of known emissivity of 0.92. Specimens were not coated when tested under ISO 834 therefore literature data were used for the emissivities (see Table 4). The convective heat transfer coefficient were calculated using formulation from Incropera and Dewitt [28] for the unexposed side and the exposed side in the radiant panel tests. The convective heat transfer coefficient for the exposed side in the furnace tests is used as specified in Eurocode 1 [29]. The uncertainties linked to this assumptions are not under the scope of this study but have been investigated elsewhere [4, 5].
3.2 Heat and Mass Transfer in Stone Wool Exposed to ISO 834
Stone wool is a porous material; thus hot air can penetrate through it. Yet, if it is cladded by an impermeable material there is a limitation of the air coming through. Hence, different behaviour can be expected depending on the properties of each layer of the composite. This was observed by Andres et al. [24] where samples of the same stone wool with and without stainless steel cladding were exposed to ISO 834 temperature–time exposure. In order to model the effect of hot air passing through the stone wool, a simplified mass transfer model is implemented by:
-
Assigning a pressure boundary of 20 Pa on the exposed side, which is the target pressure difference between the inside and outside of the furnace in standard ISO 834 fire testing for horizontal specimens.
-
Calculating the velocity of the flow by Darcy’s law (Eq. 5)
$${\text{u}}_{{\text{g}}} = { } - \frac{{\upkappa }}{{{\upmu }_{{\text{g}}} }}\frac{{\partial {\text{p}}_{{\text{g}}} }}{\partial x}$$(5)the permeability of the stone wool \({\upkappa }\) is assumed as 10–9 m2, and \({\upmu }_{{\text{g}}}\) is the viscosity of the air at atmospheric pressure (Pa∙s).
-
The heat transfer in the wool is calculated with heat transfer in porous media, where it is assumed there are a solid and a gas phase. The velocity in the gas phase is then coupled with conservation of energy (Eq. 6)
$$\left( {\rho c_{p} } \right)_{{eff}} \frac{{\partial T}}{{\partial t}} + \frac{\partial }{{\partial x}}\left( { - k_{{eff}} \frac{{\partial T}}{{\partial x}}} \right) - \rho_{g} c_{{p,g}} \frac{\partial }{{\partial x}}\left( {u_{g} \frac{{\partial T}}{{\partial x}}} \right) = 0$$(6)The effective thermal conductivity and effective specific heat consider only the apparent stone wool properties. The first and second term represent the energy storage and the conduction in the wool with the effective thermal properties of the stone wool. The third term accounts for the convection in the porous due to the hot air passing through.
3.3 Materials Properties and Sub-Models
3.3.1 Stainless Steel
Stainless steel is a material of high thermal conductivity, whose thermal properties are well defined in literature [32]. In Fig. 2 the thermal conductivity and specific heat of stainless steel are plotted as reported in Eurocode 3: Part 1–2 [32]. The density of stainless steel may be considered constant with temperature with a value of 7850 kg/m3.
Thermal conductivity and specific heat of stainless steel [32]
3.3.2 Stone Wool
Stone wool is an insulation material extensively used in building and marine applications [33, 34]. It is composed of inorganic mineral fibres and a small percentage of organic binder and water repellent oils. The heat transfer through the wool is a combination of conduction through the fibres, and gas phase conduction and radiation in the voids [35, 36]. However, accounting for these phenomena in the wool separately is not feasible and apparent thermal properties are commonly used. Several authors have reported the thermal properties of stone wool at elevated temperatures [23, 37,38,39]. Moreover, the small content of organic binder undergoes exothermic reactions when exposed to heat. Thermo-gravimetric analysis [23, 39,40,41,42] have shown two exothermic reactions occurring between 200 and 600°C that correspond to the pyrolysis and oxidative combustion of the small percentage in weight of organic content, and a third exothermic reaction occurring after 700°C that corresponds to the crystallization of the fibres. These reactions potentially increase the temperature within the wool as shown by Livkiss et al. [23] and Olsen et al. [38]. The stone wool thermal properties used for modelling purposes were retrieved from Livkiss et al. [23]. A summary is presented in Fig. 3 and Table 5. There are three different stone-wools involved in the study, two are higher density wool commonly found in steel sandwich panels (SW1 and SW2), and one is low-density wool common in gypsum wall assemblies (SW3).
Thermal conductivity as reported by Livkiss et al. [23] and used for modelling
The specific heat is taken as a constant value of 840 J/kgK according to Keerthan and Mahendran [8]. The thermal conductivity values reported by Livkiss et al. [23] for low density wool (Test 1–2) and highly dens stone wool (Test 3–4) are plotted in Fig. 3 together with the simplified values used for modelling in this study. A permeability of 10–9 m2 was assumed for stone wool, which is in the same order of magnitude as the one provided by other authors [43].
The exothermic reaction occurring in the binder is modelled using the Arrhenius formulation (Eq. 7). The energy provided by the reactions (Eq. 8) in the wool is added as an additional heat source in Eq. 1. Livkiss et al. [23] reported Ea and A parameters for three different wools obtained using TGA and MCC data. Livkiss et al. also reported the total heat of combustion for three different types of wool, having an average of 0.9 MJ/kgwool, Sjöström and Jansson [39] reported a value of 0.6 MJ/ kgwool, for a stone wool density of 170 kg/m3.
Using the different Arrhenius values for the reaction kinetics properties (pre-exponential constant A and activation energy Ea) as reported by Livkiss et al. [23] leads to very similar results. Therefore, the same values of A and Ea were used for the three different stone wools. Table 6 shows the assumed values for modelling the combustion of organic content in the wool.
Two hypothesis are used to model the energy released by combustion of the organic content of the wool:
-
1.
The rate of energy released by the wool is not limited
-
2.
The rate of energy released by the wool is limited (see Table 2).
The reason behind limiting the amount of energy released in the stone wool is that the parameters describing the reactions inside the wool are obtained from micro-scale tests where all the oxygen necessary to undergo the combustion is provided. However, this is not always the case in larger scale experiments. Hypothesis 2 is used to account for the effect of the oxygen availability, for that purpose, different limits of amount of energy released are set as a fitted input parameter (without physical meaning). The limits set in this are study are 45, 90 and 180 kW/m3. A similar approach was followed by Livkiss et al. [23] to model slug calorimetry tests with micro-scale data, being the limit in this case 46.7 kW/m3.
3.3.3 Gypsum plasterboard
Gypsum plasterboards are extensively used in building applications as interior or exterior claddings. One of the main reasons for their vast presence in the building sector is their fire properties [7]. Gypsum plasterboards are composed of calcium sulfate dihydrate that calcines in a two-step endothermic reaction releasing its chemically bound and free water [7]:
The first reaction (Eq. 9) occurs approximately at 120°C and the second (Eq. 10) around 200°C [11, 44], depending on the heating conditions and composition of the board [45]. These endothermic reactions release water in the liquid phase and there is extra energy required to evaporate the water (Q3) [14, 46]. A third exothermic reaction (Eq. 11) occurring at higher temperatures is linked to the transformation of calcium sulfate from its soluble form to insoluble form (around 400°C [44, 47, 48]). Some authors report a fourth reaction due to the decarbonation of calcium carbonate, present in the gypsum as an impurity [13, 44, 46, 49]. This reaction occurs between 650 and 700°C, and it is not always detected due to the low content of calcium carbonate in gypsum plasterboards.
The thermal properties of gypsum plasterboard have been reported by numerous studies [37, 48, 50,51,52,53,54], including thermal conductivity, specific heat, coefficient of thermal expansion, density and density reduction. The values provided by different authors differ in many cases due to different testing methods and compositions of the tested boards. Nevertheless, there is a general trend where DSC energy measurements on gypsum plasterboards show two endothermic peaks attributed to the dehydration reaction and a third exothermic peak corresponding to the decarbonation. In many cases this reaction is disregarded for modelling purposes. Likewise, the thermal conductivity drops until 200°C, above this value the thermal conductivity increases. Figure 4 shows the thermal conductivity and specific heat as reported by Rahmanian [51] for modelling purposes, where the third and fourth reactions is disregarded.
Thermal conductivity (left) and specific heat (right) of gypsum plasterboard [51]
Two different gypsum plasterboards were used in the composite testing reported by Andres et al. [24]. Table 7 shows their ambient thermal properties. G1 corresponds to a standard wallboard whereas G2 is fibre reinforced plasterboard usually found in exterior partitions. Values in Table 7 together with the thermal conductivity as a function of temperature plotted in Fig. 4 are used in the modelling.
The reactions occurring in gypsum plasterboard have been characterised using Arrhenius formulation (Eq. 12) by several authors [14, 46, 55, 56].
where \(\alpha\) is the conversion fraction of the reaction (the mass percentage of the reactants that varies between one and zero), \(\frac{d\alpha }{{dt}}\) is the rate of conversion or rate of reaction, and f(α)n is the reaction model. Different authors have lumped together or disregarded reactions in order to simply the models. Values for the Arrhenius parameters reported by some authors are presented in Table 8. Craft et al. [14] only consider one dehydration reaction. Kukuck [55] considers two dehydration reactions and a third exothermic reaction corresponding to the transformation of \({ }CaSO_{4}\) soluble to the insoluble form. Janssens [46] initially considers two reactions, the first one being the dehydration, and the second one being the decarbonation which occurs at 700 °C. Later he divides the first reaction into two overlapping reactions. Kolaitis and Founti [56] consider the two dehydration reactions.
Two different approaches are followed to model the dehydration reactions of gypsum plasterboard:
-
1. Reactions are taken into account with a temperature dependent specific heat (Fig. 4). This corresponds to Model 3A in Table 2 and 3.
-
2. Arrhenius modelling of the dehydration reactions. The Arrhenius parameters are taken from Kukuck [55] (Table 8) and a constant specific heat value of 950 J/kg·K is used. This corresponds to Model 3Ac. Kukuck’s reaction scheme was chosen after a sensitivity analysis.
Gypsum plasterboards are coated with a paper lining that provides strength and protects the gypsum. This paper lining is difficult to detach and in many cases the properties of gypsum plasterboards include properties of the gypsum and the paper lining. Park et al. [11] reported that the specific heat of gypsum plasterboard was around 14% higher with paper lining than without at room temperature. Additionally, it has been hypothesized that the paper lining might burn and thus increase the heat exposure in certain situations [14]. Bhargava et al. [57] performed TGA tests to the paper lining of gypsum plasterboards. Based on the data presented in Bhargava et al. [58] the Ahrrenius parameters presented in Table 9 were obtained (see supplementary document for further description). The energy released due to the reaction of the paper lining is included in Model 3Ad. For modelling purposes, the heat of combustion of paper was taken as the heat of combustion of cellulose.
4 Results
The results of the modelling versus experimental data are plotted in Figs. 5–7. The experimental data is ploted from two repeated tests for the steel composites (Figs. 5, 6). In Fig. 5 the unexposed side results for S-SW-S composites are plotted for the four different heat exposures described in Tables 1, 2 and 3. The modelling results show that the simple heat transfer (Model 1A) can predict the temperatures on the unexposed side of the specimen well at the 7 kW/m2, variable and ISO 834 exposures. At 60 kW/m2 the model over-predicts the results. However, including the effect of the combustion of the organic content of the wool does not provide better results. Combustion of the organic content without limiting the energy released in the reaction (Model 1Aa) results in over-estimations of the results, resulting in an initial peak in temperature above 325 °C for all heat exposures except 7 kW/m2. Regarding Model 1Ab with a limiting energy release to 45 kW/m3 (higher limiting factors will lead to higher energy released and thus higher temperatures) the results are better than without limitation, however Model 1A still preforms better. This indicates that the shielding effect of the steel plate on the exposed side is preventing the passage of hot air through the wool, limiting the combustion seen in micro-scale or delaying it to later stages.
The results of stone wool-steel composite case are plotted in Fig. 6. The modelling results are plotted against two repeated tests. In this case the cladding on the exposed side is omitted, thus the passage of hot air through the construction is not limited. In the experimental results a temperature peak is observed after 15 min. The tests were performed in a reduced scale furnace and air was constantly being pumped into the combustion chamber [24]. Therefore, this peak is most likely associated to the combustion of the organic content of the wool. Figure 6a plots the results of heat transfer modelling including only heat transfer (Model 2A), and Fig. 6b presents the results of the heat and mass transfer modelling (Model 2B). Models 2Aa and 2Ba, that include the combustion of the organic content without limiting effect, result in the peak temperature to occur faster and at a higher magnitude than observed in the tests. On the other hand, Model 2Ab with limit 1, limit 2 and limit 3 (46, 90 and 180 kW/m3 respectively) are not capable of reproducing the peak either, because they over-estimate the temperature. However, Model 2Bb that accounts for the passage of hot air through the specimen and limits the amount of energy released is capable of capturing the peak, and the best fit is retrieved when the released energy is limited to 180 kW/m3. This model results in the best predictions of the temperature increase during the first 10–20 min. After that point, the inclusion of mass transfer in the modelling does not provide any extra benefit.
The results for gypsum-stone wool- gypsum composites are plotted in Fig. 7. The backside temperatures of the exposed board (exp) and the unexposed side temperatures (unexp) are included in the figure to better present the delay in temperature raise due to the calcination reactions in gypsum plasterboard. The test results are plotted together with modelling results from Models 3A. Test data from only one experiment is included for simplicity of the plots. Modelling the dehydration reactions by including them in the specific heat value (Model 3A) or using Arrhenius equation (Model 3Ac) does not result in much different predictions on the unexposed side temperatures. Yet, Arrhenius kinetic model provides closer results to the experimental in the backside temperature of the exposed board, especially at low incident heat exposure of 7 kW/m2. Model 3Ab includes the combustion of the binder modelled with kinetic reactions and with no limit of the amount of energy released. The temperature peak on the exposed side in the 60 kW/m2 and variable heat exposure is captured by the modelling. This peak is not observed at 7 kW/m2 during testing as no reaction or limited reaction of the organic content is expected at such low heating exposures. On the other hand, at ISO 834 exposure, no peak is observed either. The authors link this to the low oxygen content inside the furnace to undergo combustion. These tests were performed in a conventional oven where no extra air is being pumped into the chamber. In Model 3Ad the effect of burning of the paper is included as an energy released, considering the Arrhenius parameters shown in Table 9 and a heat of combustion of cellulose. However, the predicted energy released is so low that it does not influence the modelling results. None of the models are capable of reproducing the initial raise on the unexposed side temperature. This fast increase might be associated to water evaporation-condensations cycles from the water released from gypsum plasterboards, and these are not included in the studied models.
The performance of the models with respect to the measured test data is evaluated according to the following points:
-
The temperature prediction at the end of the test is evaluated as the percentage of temperature deviation between the model and the test data
-
The early stage temperature predictions is evaluated as percentage of temperature deviation between the model and the test data at 10 min since the start of the test
-
The ability of the model to predict local peaks is evaluated for stone wool-steel composite as the local maximum temperature and time to reach this temperature.
Tables 10, 11, and 12 show the deviations between the unexposed side temperature model predictions and the test data for S-SW-S, SW-S and G-SW-G composites respectively. The deviations on unexposed side temperatures at the end of the test are below 30% in all cases, whereas at 10 min the deviations are much larger. This difference is more pronounced in G-SW-G composites, where in early stages there might be an evaporation–condensation phenomena that is not being taken into account in the model. Table 13 shows the peak temperatures and time to peak for stone wool-steel composites and the deviation between test data and models. A table showing a qualitative summary of the models used in this study and their ability to predict early stage unexposed temperature, final unexposed temperature predictions and local peaks predictions can be found in the appended documentation. From the analysis of the temperature deviations it is observed that:
-
For S-SW-S composite, including the combustion of the binder in the model does not provide significantly better modelling predictions. Table 10 shows negligible difference between the deviations.
-
For S-SW-S composite, heat transfer model are capable of predicting early unexposed side temperature for S-SW-S composites only at low heat exposure (7 kW/m2), showing a difference of 14% or less.
-
For SW-S composites, heat and mass transfer models provide significant better modelling predictions at early stages of the modelling and of the peak temperatures when the combustion of the organic content is included. Tables 11 and 13 show the difference between the models and the test data.
-
For SW-S, peak temperatures and time to peak predictions are best achieved when modelling heat and mass transfer limiting the amount of energy released by the combustion of the organic content to 180 kW/m3.
-
For G-SW-G composites, considering the calcination reactions of gypsum in the specific heat term or using Arrhenius formulation yields to similar results on the unexposed side temperature at the end of the test, being the largest deviation at 7 kW/m2
-
For G-SW-G composites, the model prediction are closer to the test data at the end of the tests. Deviation at the end of the tests vary from 7–25%, whereas at 10 min vary from 7 to 75%.
-
Unexposed side temperature predictions of the exposed gypsum board on G-SW-G composites are able to predict local peaks when including the combustion of the binder for the 60 kW/m2 and variable heat exposure.
5 Discussion
The models presented in this study are one-dimensional. This assumption was validated by experimental and numerical data. The temperature difference measured by thermocouples placed in different locations during the test ensured that the heat was being transferred in one main direction [24]. Additionally, the one-dimensional heat transfer model was compared to two-dimensional to ensure that in the central area of the specimens a one-dimensional heat transfer assumption was sufficient. The analysis of the one-dimensional heat transfer phenomena (experimentally and numerically validated) allows to forsee if adding further phenomena to the model provide more accurate temperature predictions. However, when trying to model larger scale composites phenomena, such as mechanical degradation of boards (e.g. cracking and falling-off of boards), thermal bowing of studs, opening of gaps in insulation material, will limit the applicability of one dimensional heat transfer models.
Results have shown that for steel-stone wool-steel composites the inclusion of the energy released due to combustion of the organic content of the wool does not provide more accurate results. However, in the situation where the stone wool is directly exposed to the heat and there is enough oxygen flow a peak in temperatures is detected. This was also observed by Paudel et al. [57]. In this case, the inclusion of a pressure driven mass flow and the combustion of the organic content provides better approximation to the test results. However, as already observed by Livkiss et al. [23], the amount of energy being released by the reaction is smaller than what occurres at micro-scale level.
Modelling the calcination reaction in gypsum plasterboard using Arrhenius parameters or including the reaction within the specific heat values yield to similar results, and good temperature predictions on the exposed board. Previous work has already used both approaches [49, 56, 59, 60]. In this manuscript, it is also validated for different heat exposures. On the unexposed side of the G-SW-G the models are unable to capture the increase in temperature in early stages of the fire. This has been also the case in previous modelling attempts [59,60,61]. Understanding evaporation–condensation cycles and including them in the model could potentially provide better modelling predictions. Weber [31] showed that including phase change of water in the model lead to a temperature plateau around 100 °C, which could represent the plateau observed in one layer of gypsum tests under heat exposures. Further work needs to be done to validate it in composite constructions.
6 Conclusion
Models to predict the unexposed side temperature of stone wool layered composites with stainless steel or gypsum plasterboard claddings have been presented and compared against experimental data. The different constructions types and heating conditions were chosen in order to represent a range of different scenarios, which in turn can be used to study the suitability of increasing the complexity of the heat transfer model by inclusion of different sub-models. A series of kinetic reaction sub-models obtained from micro-scale data define: (1) the combustion of the organic binder content of the wool, (2) the calcination reactions in the gypsum plasterboard, (3) burning of the paper of gypsum plasterboard. Additionally, material thermal properties obtained from bench scale test data, for stone wool and gypsum plasterboard, are used as opposed to traditional fitting input parameters. The models are compared against test data for four different heat exposures: (1) 7 kW/m2 constant incident heat exposure, (2) 60 kW/m2 constant incident heat exposure, (3) a variable heat exposure between 4.6 and 62 kW/m2, and (4) ISO 834 temperature time exposure.
Stone wool is a permeable insulation material, and its behaviour in fire exposures has shown to be affected by the cladding material or the lack of cladding. At bench scale testing, the small percentage in weight of organic content has shown a combustion reaction leading to an increase of temperature within the wool. However, at a larger scale its behaviour has shown to be very much dependent on the permeability of hot gases through the cladding material. Three different physical processes are included in the modelling: heat transfer, heat and mass transfer, and internal combustion of the organic content of the wool with and without limiting the amount of energy released during that combustion. It is observed that the inclusion of combustion of the organic content in the wool is not providing better modelling results if the stone wool is protected by a steel cladding. The impermeable nature of steel might prevent the passage of hot air, limiting the amount of oxygen available to undergo the combustion. On tests performed on stone wool-steel composites without cladding, in an oven where ambient air is being pumped into the chamber, a peak in temperature is observed that could be linked to the reaction of the organic content of the wool. However, models developed from micro-scale data predict faster and higher peak than experimentally observed. Including the combustion of the binder with limited amount of energy released model when modelling gypsum-stone wool-gypsum composite tested at 60 kW/m2 and a variable exposure provides good estimation of peak temperatures. In these tests the samples were placed in an open space and they were heated up with a radiant panel (H-TRIS). Due to the permeable nature of gypsum and the availability of oxygen in the air, the binder in the wool could combust and the model captured this process. However, this phenomenon was not observed in the ISO 834 exposure, which most likely was due to the low oxygen content during the tests. A better understanding of how combustion reactions in the wool take place under different conditions is necessary in order to obtain more accurate models. This poses the question of what would be the case in a real fire scenario, and whether a low oxygen scenario should be accounted for or not in this type of modelling. When mass transfer is added to the heat transfer it provides better predictions of initial temperature increase in the stone wool-steel composites without protective steel cladding on the exposed side. Modelling the dehydration reaction of gypsum plasterboards can successfully be done, either by considering the reactions in the specific heat or by using an Arrhenius kinetic model. However, the used Arrhenius model provides better predictions at low heat exposures. An evaporation–condensation model is considered to be needed in order to capture the initial increase of temperature of gypsum plasterboard.
The novelty of the paper lies both in the different physical process included in the models, increasing the model complexity, and the different heat exposures. This study shows how material properties and reactions schemes obtained from reduced scale testing can be used to achieve good modelling predictions. However, there is a need for better understanding on the environmental conditions that materials are exposed to in a fire event, further validation of the models, and quantification of experimental versus modelling uncertainties. Modelling tools such as the one provided in this study can help manufacturers understand better the performance of their products in fire, and can work as development tools in order to quantify how changes in the construction can impact the overall fire performance.
Abbreviations
- \(A\) :
-
Arrhenius pre-exponential factor (1/s)
- cp :
-
Specific heat (J/kgK)
- \(CaSO_{4}\) :
-
Calcium sulfate
- \(dH\) :
-
Heat of combustion (kJ/kg)
- DSC:
-
Differential scanning calorimetry
- \(\frac{\text{d}\alpha}{\text{d}t}\) :
-
Rate of conversion (1/s)
- \(E_{a}\) :
-
Activation Energy (J/mol)
- f(α)n :
-
Reaction Model
- G:
-
Gypsum
- hc :
-
Heat transfer coefficient (W/m2K)
- H-TRIS:
-
Heat Transfer Rate Inducing System
- \(H_{2} O\) :
-
Water
- ISO:
-
International Organization for Standarization
- k:
-
Thermal conductivity (W/mK)
- \(m\) :
-
Mass (kg)
- MCC:
-
Micro-combustion calorimetry
- \({\text{p}}_{{\text{g}}}\) :
-
Pressure (Pa)
- \(\dot{q}_{{}}^{^{\prime\prime}}\) :
-
Net heat flux (kW/m2)
- \(Q_{{}}\) :
-
Energy released by the reaction (kW)
- R:
-
Universal gas constant (8.314 J/molK)
- S:
-
Steel
- SW:
-
Stone wool
- T:
-
Temperature (K or °C)
- t:
-
Time (s)
- TGA:
-
Thermogravimetric analysis
- ug :
-
Velocity of the air (m/s)
- α:
-
Absorptivity (-) or conversion (-)
- \({\uprho }\) :
-
Density (kg/m³)
- \({\upvarepsilon }_{{}}\) :
-
Emissivity of the material (-)
- \({\upsigma }\) :
-
Stefan-Boltzmann constant (5.6704·10−8 W/m2K4)
- \({\upkappa }\) :
-
Permeability of the stone wool (m2)
- \({\upmu }_{{\text{g}}}\) :
-
Viscosity of the air at atmospheric pressure (Pa·s)
References
International Organisation for Standardisation (1999) ISO 834–1:1999: Fire Resistance Tests – Elements of Building Construction – Part 1: General Requirements
CEN(1999) CEN, EN 1363–2:1999, Fire Resistance tests alternative and additional procedures
Harmathy TZ (1965) Ten rules of fire endurance rating. Fire Technol 1(2):93–102. https://doi.org/10.1007/BF02588479
Livkiss K, Andres B, Johansson N, Van Hees P (2015) Uncertainties in material thermal modelling of fire resistance tests. In: European symposium of Fire Safety Science. p. 1–6
Livkiss K, Andres B, Johansson N, van Hees P (2017) Uncertanties in modelling heat transfer in fire resistance tests: a case study of stone wool sandwich panels. Fire Mater. https://doi.org/10.1002/fam.2419
Keerthan P, Mahendran M (2012) Numerical modelling of non-load-bearing light gauge cold-formed steel frame walls under fire conditions. J Fire Sci 30(5):375–403. https://doi.org/10.1177/0734904112440688
Thomas G (2002) Thermal properties of gypsum plasterboard at high temperatures. Fire Mater 26(1):37–45. https://doi.org/10.1002/fam.786
Keerthan P, Mahendran M (2012) Thermal performance of composite panels under fire conditions using numerical studies: plasterboards, rockwool, glass fibre and cellulose insulations. Fire Technol 49(2):329–356. https://doi.org/10.1007/s10694-012-0269-6
Andres B, van Hees P (2015) Experimental and Thermal Analysis of Wall Assemblies Exposed to Standard and Parametric Fires. Conference Proceedings. CONFAB
Ghorbani Z, Webster R, Lázaro M, Trouvé A (2013) Limitations in the predictive capability of pyrolysis models based on a calibrated semi-empirical approach. Fire Saf J 61:274–288. https://doi.org/10.1016/j.firesaf.2013.09.007
Park S, Manzello S, Bentz DP, Mizukami T (2009) Determining thermal properties of gypsum board at elevated temperatures. Fire Mater. https://doi.org/10.1002/fam.1017
Olsen H, Sjöström J, Jansson R, Anderson J (2013) Thermal properties of heated insulation materials. proceedings of. 13th International Fire Engineering Conference, Interflam. 1049–1060
Kontogeorgos DA, Founti MA (2012) Gypsum board reaction kinetics at elevated temperatures. Thermochim Acta 529:6–13. https://doi.org/10.1016/j.tca.2011.11.014
Craft ST, Isgor B, Hadjisophocleous G, Mehaffey JR (2008) Predicting the thermal response of gypsum board subjected to a constant heat flux. Fire Mater 32(6):333–355. https://doi.org/10.1002/fam.971
Torero JL (2013) Scaling-Up fire. Proc Combust Inst 34(1):99–124. https://doi.org/10.1016/j.proci.2012.09.007
Camillo A (2013) Multi-scale investigation of fire behaviour of a seat and a wall panel from European railway transport system
Rogaume T (2019) Thermal decomposition and pyrolysis of solid fuels: objectives, challenges and modelling. Fire Saf J 106:177–188. https://doi.org/10.1016/j.firesaf.2019.04.016
Stoliarov SI, Li J (2016) Parameterization and validation of pyrolysis models for polymeric materials. Fire Technol 52(1):79–91. https://doi.org/10.1007/s10694-015-0490-1
Richter F, Rein G (2020) A multiscale model of wood pyrolysis in fire to study the roles of chemistry and heat transfer at the mesoscale. Combust Flame 216:316–325. https://doi.org/10.1016/j.combustflame.2020.02.029
Kodur V, Harmathy TZ (2002) Properties of Building Materials. In: Philip J. DiNenno, P.E. (Hughes Associates, Inc.) E-C, editor. SFPE Handbook of Fire Protection Engineering. SFPE; . p. 165–191
Bal N, Rein G (2013) Relevant model complexity for non-charring polymer pyrolysis. Fire Saf J 61:36–44. https://doi.org/10.1016/j.firesaf.2013.08.015
Huang X, Li K, Zhang H (2017) Modelling bench-scale fire on engineered wood: effects of transient flame and physicochemical properties. Proc Combust Inst 36(2):3167–3175. https://doi.org/10.1016/j.proci.2016.06.109
Livkiss K, Andres B, Bhargava A, van Hees P (2018) Characterization of stone wool properties for fire safety engineering calculations. J Fire Sci. https://doi.org/10.1177/0734904118761818
Andres B, Livkiss K, Hidalgo JP, van Hees P, Bisby L, Johansson N et al (2018) Response of stone wool insulated building barriers under severe heating exposures. J Fire Sci. https://doi.org/10.1177/0734904118783942
Comsol (2013) COMSOL Multiphysics Reference Manual
Wickström U (2016) Temperature Calculation in Fire Safety Engineering. Springer. https://doi.org/10.1007/978-3-319-30172-3
Maluk C, Bisby L, Krajcovic M, Torero JL (2016) A heat-transfer rate inducing system ( H-TRIS ) test method. Fire Saf J. https://doi.org/10.1016/j.firesaf.2016.05.001
Incropera FP, DeWitt DP, Bergman TL, Lavine AS (2007) Fundamentals of Heat and Mass Transfer
CEN (2002) Eurocode 1: Actions on structures. Part 1–2 General Actions- Actions on structures exposed to fire. 2011;2
Paloposki T, Liedquist L (2005) Steel emissivity at high temperatures
Weber B (2012) Heat transfer mechanisms and models for a gypsum board exposed to fire. Int J Heat Mass Transf 55(5–6):1661–1678. https://doi.org/10.1016/j.ijheatmasstransfer.2011.11.022
CEN (2005) Eurocode 3: Design of Steel Structures. Part 1–2: General Rules - Structural Fire Design
Papadopoulos AM, Karamanos A, Avgelis A. Environmental Impact of Insulating Materials At the End of Their Useful Lifetime.
Hidalgo-Medina JP (2015) Performance based methodology for the fire safe design of insulation materials in energy efficient buildings. PhD Thesis, University of Edinburgh
Kumaran MK, Stephenson DG (1986) Heat Tansport Through Thermal Insulation: An Application of the Principles of Thermodynamics of Irreversible Processes. Ottawa
Dyrbøl S (1998) Heat transfer in Rockwool modelling and method of measurement. PhD Thesis, Danish Technical University
Bénichou N, Sultan MA (2005) Thermal properties of lightweight-framed construction components at elevated temperatures. Fire Mater 29:165–179. https://doi.org/10.1002/fam.880
Olsen H, Jansson R, Anderson J (2013) Thermal Properties of Heated Insulation Materials. I Proceedings of. 13th International Fire Engineering Conference, Interflam. p. 1049–1060
Sjöström J, Jansson R (2012) Measuring thermal material properties for structural fire engineering. In: 15th International Conference on Experimental Mechanics
Andres B, Hidalgo JP, Bisby L, Hees P Van (2017) Experimental analysis of stone wool sandwich composites exposed to constant incident heat fluxes and simulated parametric fires. Proceedings of Fire and Materials Conference. San Francisco, USA
N N, Didomizio M, Weckman EJ, Roos R (2017) Determination of thermochemical properties of stone wool insulation materials. In: Proceedings of Fire and Materials Conference. San Francisco, USA
Hidalgo JP, Torero JL, Welch S (2016) Experimental characterisation of the fire behaviour of thermal insulation materials for a performance-based design methodology. Fire Technol. https://doi.org/10.1007/s10694-016-0625-z
Dyrbøl S (1998) Heat transfer in Rockwool modelling and method of measurement. Part I: the effect of natural convection on heat transfer in fibrous materials
Melinge Y, Lanos C, Nguyen KS, Daiguebonne C, Guillou O, Freslon S (2011) One-dimensional-time study of the dehydration of plasterboards under standard fire condition (ISO 834): thermo-chemical analysis. J Fire Sci 29(4):299–316. https://doi.org/10.1177/0734904110391889
Ghazi Wakili K, Koebel M, Glaetti T, Hofer M (2015) Thermal conductivity of gypsum boards beyond dehydration temperature. Fire Materials. https://doi.org/10.1002/fam.2234
Janssens M (2011) Thermogravimetric Study of Dehydration and Thermal Degradation of Gypsum Board at Elevated Temperatures. 10:295–306. https://doi.org/10.3801/IAF
Park S, Manzello S, Bentz DP, Mizukami T (2009) Determining Thermal Properties of Gypsum Board at Elevated Temperatures. Fire Materials. https://doi.org/10.1002/fam
Kontogeorgos D, Founti M (2010) Numerical investigation of simultaneous heat and mass transfer mechanisms occurring in a gypsum board exposed to fire conditions. Appl Therm Eng 30(11–12):1461–1469. https://doi.org/10.1016/j.applthermaleng.2010.03.006
Ghazi Wakili K, Hugi E, Wullschleger L, Frank T (2007) Gypsum board in fire - Modeling and experimental validation. J Fire Sci 25(3):267–282. https://doi.org/10.1177/0734904107072883
Kolarkar PN (2010) Structural and Thermal Performance of Cold-formed Steel Stud Wall Systems under Fire Conditions. PhD Thesis, University of Queensland
Rahmanian I (2011) Thermal and Mechanical Properties of Gypsum Boards and their Influences on Fire Resistance of Gypsum Board Based Systems. PhD Thesis, University of Manchester
Sultan M, Alfawakhiri F, Benichou N (2001) A model for predicting heat transfer through insulated steel-stud wall assemblies exposed to fire. Fire Materials Conference Proceedings. 495–506
Bénichou N, Sultan MA (2005) Thermal properties of lightweight-framed construction components at elevated temperatures. Fire Materials 29(3):165–179. https://doi.org/10.1002/fam.880
Semitelos GK, Mandilaras ID, Kontogeorgos DA, Founti MA (2014) Simplified correlations of gypsum board thermal properties for simulation tools. Fire Materials. https://doi.org/10.1002/fam
Kukuck S (2009) Heat and mass transfer through gypsum partitions subjected to fire exposures, NIST IR 7461. National Insitute of Standards and Technology, US Department of Commerce
Kolaitis DI, Founti MA (2013) Development of a solid reaction kinetics gypsum dehydration model appropriate for CFD simulation of gypsum plasterboard wall assemblies exposed to fire. Fire Saf J 58:151–159. https://doi.org/10.1016/j.firesaf.2013.01.029
Paudel D, Rinta-Paavola A, Mattila HP, Hostikka S (2020) Multiphysics modelling of stone wool fire resistance. Fire Technol. https://doi.org/10.1007/s10694-020-01050-5
Bhargava A, Andersson B, Hees P Van, Sina H, Iyengar S, Technology S (2016) Distributed Reactivity Model to Predict Multistage Pyrolysis of Polymeric Materials and Sensitivity Analysis. Interflam’16, 14th International Conference and Exhibition on Fire Science and Engineering. 4–6th July, London, UK: p. 107–118
Bruns M, Prasad K (2014) Parametric analysis of heat transfer in gypsum wallboard partitions. Fire Safety Science 11:598–611. https://doi.org/10.3801/IAFSS.FSS.11-598
Craft S, Isgor B, Mehaffey J, Hadjisophocleous G (2008) Modelling heat and mass transfer in wood-frame floor assemblies exposed to fire. Fire Safety Science 9:1303–1314. https://doi.org/10.3801/IAFSS.FSS.9-1303
Kontogeorgos D, Ghazi Wakili K, Hugi E, Founti M (2012) Heat and moisture transfer through a steel stud gypsum board assembly exposed to fire. Constr Building Materials 26(1):746–754. https://doi.org/10.1016/j.conbuildmat.2011.06.083
Acknowledgments
This project was done thanks to the support of the European Union’s Seventh Framework Program under grant no. 316991. This is study is part of a collaborative project between Lund University and the Danish Institute of Fire and Security Technology, called FIRETOOLS.
Funding
Open access funding provided by Lund University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Andres, B., Livkiss, K., Bhargava, A. et al. Using Micro-Scale and Solid Material Data for Modelling Heat Transfer in Stone Wool Composites Under Heat Exposures. Fire Technol 57, 1541–1567 (2021). https://doi.org/10.1007/s10694-021-01122-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10694-021-01122-0