Abstract
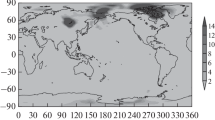
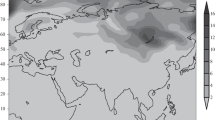
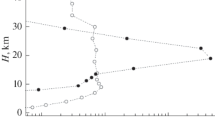
The results of one-dimensional calculations of the height profiles of nucleated sulfate aerosol particles for the northern mid-latitudes and tropics in winter are presented. Numerical calculations were performed using a three-dimensional model of the transport and transformation of multicomponent gas and aerosol substances in the atmosphere, incorporating photochemistry, nucleation involving neutral molecules and ions, as well as condensation/evaporation and coagulation. It is found that the resulting dynamics of the formation of aerosol particle nuclei is not a simple sum of ion and binary (water vapor/sulfuric acid) nucleation rates. This dynamics is determined by the ratio of critical radii of nucleated particles due to binary and ion nucleation of these substances (rcr_bin and rcr_ion) depending on temperature, relative humidity, and ionization rate. This should be taken into account in modeling the gas and aerosol composition of the atmosphere and comparing calculated and observed data.


Similar content being viewed by others
REFERENCES
A. E. Aloyan, A. N. Yermakov, and V. O. Arutyunyan, “Aerosols in the Troposphere and Lower Stratosphere. Sulfate Particles in Northern Latitudes,” Optika Atmosfery i Okeana, No. 2, 31 (2018) [in Russian].
A. E. Aloyan, A. N. Yermakov, and V. O. Arutyunyan, “Modeling the Impact of Ions on the Dynamics of Atmospheric Aerosol Formation,” Izv. Akad. Nauk, Fiz. Atmos. Okeana, No. 1, 57 (2021) [in Russian].
A. E. Aloyan, A. N. Yermakov, and V. O. Arutyunyan, “Sulfate Aerosol Formation in the Troposphere and Lower Stratosphere,” in The Investigation of Possible Stabilization of Climate Using New Technologies (Roshydromet, Moscow, 2012) [in Russian].
H. Akimoto, Atmospheric Reaction Chemistry (Springer Japan, 2016).
A. E. Aloyan, “Mathematical Modeling of the Interaction of Gas Species and Aerosols in Atmospheric Dispersive Systems,” Russ. J. Num. Anal. Math. Model., No. 1–4, 15 (2000).
A. D. Clarke, D. Davis, V. N. Kapustin, F. Eisele, G. Chen, I. I. Paluch, D. Lenschow, A. R. Bandy, D. Thornton, K. Moore, L. Mauldin, D. Tanner, M. Litchy, M. A. Carroll, J. Collins, and G. Albercook, “Particle Nucleation in the Tropical Boundary Layer and Its Coupling to Marine Sulfur Sources,” Science, 282 (1998).
E. E. Ferguson, “Ion–Molecule Reactions in the Atmosphere,” in Kinetics of Ion–Molecule Reactions, Ed. by P. Ausloos (Springer, Boston, 1979).
K. D. Froyd and E. R. Lovejoy, “Experimental Thermodynamics of Cluster Ions Composed of H2SO4 and H2O. 1. Positive Ions,” J. Phys. Chem., A, No. 45, 107 (2003).
U. Horrak, J. Salm, and H. Tammet, “Bursts of Intermediate Ions in Atmospheric Air,” J. Geophys. Res., 103 (1998).
M. Kanakidou, J. H. Seinfeld, S. N. Pandis, I. Barnes, F. J. Dentener, M. C. Facchini, R. van Dingenen, E. Swietlicki, J. P. Putaud, Y. Balkanski, S. Fuzzi, J. Horth, G. K. Moortgat, R. Winterhalter, C. E. L. Myhre, K. Tsigaridis, E. Vignati, E. G. Stephanou, and J. Wilson, “Organic Aerosol and Global Climate Modeling: A Review,” Atmos. Chem. Phys., No. 4, 5 (2005).
M. Kulmala, H. Vehkamaki, T. Pe, M. Dal Maso, A. Lauri, V. M. Kerminen, W. Birmili, and P. H. McMurry, “Formation and Growth Rates of Ultrafine Atmospheric Particles: A Review of Observations,” J. Aerosol Sci., 35 (2004).
Y. Kurihara and R. E. Televa, “Structure of Tropical Cyclone Developed in Three-dimensional Numerical Simulation Model,” J. Atmos. Sci., No. 5, 31 (1974).
E. R. Lovejoy, J. Curtius, and K. D. Froyd, “Atmospheric Ion Induced Nucleation of Sulfuric Acid and Water,” J. Geophys. Res., 109 (2004).
M. Noppel, H. Vehkamaki, and M. Kulmala, “An Improved Model for Hydrate Formation in Sulfuric Acid–Water Nucleation,” J. Chem. Phys., 116 (2002).
J. H. Seinfeld and S. N. Pandis, Atmospheric Chemistry and Physics. From Air Pollution to Climate Change (Wiley-Interscience, New York, 1997).
M. Wang and P. E. Penner, “Aerosol Indirect Forcing in a Global Model with Particle Nucleation,” Atmos. Chem. Phys., 9 (2009).
R. J. Weber, J. J. Marti, P. H. McMurray, F. L. Eisele, D. J. Tanner, and A. Jefferson, “Measured Atmospheric New Particle Formation Rates: Implications for Nucleation Mechanisms,” Chem. Eng. Commun., 151 (1996).
F. Yu, “Ion-mediated Nucleation in the Atmosphere: Key Controlling Parameters, Implications, and Look-up Table,” J. Geophys. Res., 115 (2010).
F. Yu, G. Luo, T. S. Bates, B. Anderson, A. Clarke, V. Kapustin, R. M. Yantosca, Y. Wang, and Sh. Wu, “Spatial Distributions of Particle Number Concentrations in the Global Troposphere: Simulations, Observations, and Implications for Nucleation Mechanisms,” J. Geophys. Res., 115 (2010).
Funding
The research was supported by the Russian Foundation for Basic Research (project numbers 18-05-00289 and 19-05-50007 (Mikromir)), as well as by the governmental assignments of Marchuk Institute of Numerical Mathematics of Russian Academy of Sciences (RAS) and the Talrose Institute for Energy Problems of Chemical Physics of RAS (theme AAAA-0047-2018-0012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Russian Text ©The Author(s), 2021, published in Meteorologiya i Gidrologiya, 2021, No. 1, pp. 53-60.
About this article
Cite this article
Aloyan, A.E., Yermakov, A.N. & Arutyunyan, V.O. The Role of Binary and Ion Nucleation of Sulfuric Acid and Water Vapor in the Dynamics of Sulfate Aerosol Formation in the Atmosphere. Russ. Meteorol. Hydrol. 46, 37–42 (2021). https://doi.org/10.3103/S1068373921010052
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068373921010052