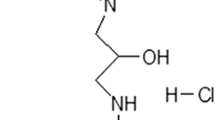
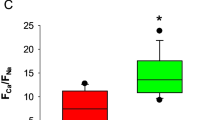
Abstract—One of the leading mechanisms of neurodegeneration in Parkinson’s disease is excitotoxicity of glutamate, the main excitatory brain neurotransmitter. Excitotoxicity develops as a result of excessive stimulation of ionotropic NMDA glutamate receptors. Drugs reversibly inhibiting NMDA receptors are considered as promising pharmacological agents that prevent the death of neurons and slow down neurodegeneration. In this work, we studied the effect of N-(2-adamantyl)-hexamethyleneimine hydrochloride (hemantane) on changes in the intracellular concentration of Ca2+ ([Ca2+]i), Na+ ([Na+]i) and mitochondrial potential (ΔΨm) induced by NMDA in cultured neurons. Cultures were prepared from the cerebral cortex of 1-2-day-old Wistar rats. Experiments with animals were performed in accordance with the ethical principles and regulatory documents recommended by the European Convention on the Protection of Vertebrate Animals. The measurements of [Ca2+]i, [Na+]i and ΔΨm were taken using a fluorescence microscopy system based on an Olympus IX-71 inverted microscope with a Sutter Lambda 10-2 multi-wavelength illumination system and a cooled CoolSnap HQ CCD camera. The system was controlled using the MetaFluor software. It was shown that short-term subtoxic doses of NMDA (2–3 min, 10 μM) cause a rapid rise in [Ca2+]i, which is reversibly inhibited by high concentrations of Mg2+ (10 mM) and hemantane (100 μM). Prolonged administration of the neurotoxic NMDA concentration (20 min, 500 μM) caused the development of delayed calcium deregulation (DCD), a steady rise in [Na+]i and a profound drop in ΔΨm. Hemantane (100 μM) reduced [Ca2+]i elevation, postponed the development of DCD, reduced mitochondrial depolarization, and helped to restore the initial values of [Ca2+]i, [Na+]i and ΔΨm after NMDA washout. Unlike hemantane, the high-affinity non-competitive inhibitor of NMDA channels MK-801 irreversibly blocked changes in [Ca2+]i and ΔΨm, even at a high NMDA concentration (500 μM). Obviously, hemantane exhibits neuroprotective properties due to a decrease in Ca2+ and Na+ fluxes through NMDA channels and a lower elevation of [Ca2+]i and [Na+]i. The reversibility of NMDA channel inhibition favors the normalization of brain functioning after termination of hemantane administration.





Similar content being viewed by others
REFERENCES
Amenta, F., Zaccheo, D., and Collier, W.L., Mech. Ageing Dev, 1991, vol. 61, no. 3, pp. 249–273.
Kolacheva, A.A., Kozina, E.A., Khakimova, G.R., Kucheryanu, V.G., Kudrin, V.S., Nigmatullina, R.R., Bazyan, A.S., Grigor’yan, G.A., and Ugryumov, M.V., Neirodegenerativnye zabolevaniya: ot genoma do tselostnogo organizma (Neurodegenerative diseases: from genome to entire body), Ugryumov, M.V., Eds., Moscow: Nauchnyi mir, 2014, vol. 1, pp. 356–422.
Nicholls, D.G., Martin, A.R., Wallace, B.D., and Fuchs, P.A., From Neuron to Brain, Sunderland: Sinauer Associates, 2001.
Sobolevsky, A.I., J. Physiol., 2015, vol. 593, no. 1, pp. 29–38.
Dirnagl, U., Iadecola, C., and Moskowitz, M.A., Trends Neurosci., 1999, vol. 22, no. 9, pp. 391–397.
Nicholls, D.G. and Budd, S.L., Physiol. Rev., 2000, vol. 80, no. 1, pp. 315–360.
Khodorov, B., Prog. Biophys. Mol. Biol, 2004, vol. 86, no. 2, pp. 279–351.
Hardingham, G.E., Biochem. Soc. Trans., 2009, vol. 37, no. 6, pp. 1147–1160.
Hardingham, G.E. and Bading, H., Nat. Rev. Neurosci., 2010, vol. 11, no. 10, pp. 682–696.
Chen, H.S.V., Pellegrini, J.W., Aggarwal, S.K., Lei, S.Z., Warach, S., Jensen, F.E., and Lipton, S.A., J. Neurosci., 1992, vol. 12, no. 11, pp. 4427–4436.
Parsons, C.G., Danysz, W., and Quack, G., Neuropharmacology, 1999, vol. 38, no. 6, pp. 735–767.
Chen, H.S.V. and Lipton, S.A., J. Neurochem., 2006, vol. 97, no. 6, pp. 1611–1626.
Stayte, S. and Vissel, B., Front. Neurosci, 2014, vol. 8.
Freitas, M.E. and Fox, S.H., Neurodegener. Dis. Manag., 2016, vol. 6, no. 3, pp. 249–268.
Kapitsa, I.G., Ivanova, E.A., Avdyunina, N.I., and Voronina, T.A., Khim.-Farm. Zh., 2019, vol. 53, no. 5, pp. 3–7.
Olivares, D.K., Deshpande, V., Shim Y.K., Lahiri, D.H., Greig, N.T., Rogers, J., and Huang, X, Curr. Alzheimer Res., 2013, vol. 9, no. 6, pp. 746–758.
Sobolevsky, A.I. and Yelshansky, M.V., J. Physiol., 2000, vol. 526, no. 3, pp. 493–506.
Elshanskaya, M.V., Sobolevskii, A.I., Val’dman, E.A., and Khodorov, B.I., Eksperim. Klin. Farmakol, 2001, vol. 64, no. 1, pp. 18–21.
Voronina, N.A., Kucheryanu, V.G., Kapitsa, I.G., and Voronina, T.A., Patogenez, 2019, vol. 17, no. 4, pp. 57–62.
Ivanova, E.A., Kapitsa, I.G., Zolotov, N.N., Val’dman, E.A., Nepoklonov, A.V., Kolyasnikova, K.N., and Voronina, T.A., Farmakokinetika i Farmakodinamika, 2016, vol. 3, pp. 9–12.
Krasil'nikova, I., Surin, A., Sorokina, E., Fisenko, A., Boyarkin, D., Balyasin, M., Demchenko, A., Pomytkin, I., and Pinelis, V., Front. Neurosci., 2019, vol. 13.
Seredenin, S.B., Voronina, T.A., Avdyunina, N.I., Morozov, I.S., Bykov, N.P., and Nerobkova, L.N., RF Patent No. 1825499, 1991.
Nerobkova, L.N., Val’dman, E.A., Voronina, T.A., Markina, N.V., and Sharkova, L.M., Eksp. Klin. Farmakol., 2000, vol. 63, no. 3, pp. 3–6.
Sharipov, R.R., Krasil’nikova, I.A., Pinelis, V.G., Gorbacheva, L.R., and Surin, A.M., Biol. Membr., 2018, vol. 35, no. 5, pp. 384–397.
Vergun, O., Keelan, J., Khodorov, B.I., and Duchen, M.R., J. Physiol., 1999, vol. 519, no. 2, pp. 451–466.
Duchen, M.R., Surin, A., and Jacobson, J., Methods Enzymol., 2003, vol. 361, pp. 353–389.
Brittain, M.K., Brustovetsky, T., Sheets, P.L., Brittain, J.M., Khanna, R., Cummins, T.R., and Brustovetsky, N., Neurobiol. D, vol. 46, no. 1, pp. 109–117.
Khodorov, B.I., Storozhevykh, T.P., Surin, A.M., Sorokina, E.G., Yuravichus, A.I., Borodin, A.V., Vinskaya, N.P., Khaspekov, L.G., and Pinelis, V.G., Biol. Membr., 2001, vol. 18, no. 6, pp. 421–432.
Genrikhs, E.E., Aleksandrova, O.P., Stel’mashuk, E.V., Novikova, S.V., Voronkov, D.N., Isaev, N.K., and Khaspekov, L.G., Annaly Klinicheskoi i eksperimental’noi nevrologii, 2019, vol. 13, no. 4, pp. 38–45.
Zinchenko, V.P., Turovskaya, M.V., Teplov, I.Yu., Bepezhnov, A.V., and Turovsky, E.A., Biofizika, 2016, vol. 61, no. 1, pp. 102–111.
Aqrawe, Z., Patel, N., Vyas, Y., Bansal, M., Montgomery, J., Travas-Sejdic, J., and Svirskis, D., PLoS One, 2020, vol. 15, no. 8.
Funding
The study was carried out according to the State tasks nos. 0520-2019-0029 and AAAA-A19-119012590191-3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare no conflicts of interest.
Ethical approval. Experiments with animals were performed in accordance with the ethical principles and regulatory documents recommended by the European Convention for the Protection of Vertebrate Animals used for Experiments (Guide for the Care and Use of Laboratory Animals: Eighth Edition. 2010), as well as in accordance with the “Rules of Good Laboratory Practice” approved by order of the Ministry of Health of the Russian Federation No. 199n of April 1, 2016.
Rights and permissions
About this article
Cite this article
Voronina, N.A., Lisina, O.Y., Krasilnikova, I.A. et al. Influence of Hemantane on Changes in Ca2+ and Na+ Caused by Activation of NMDA Channels in Cultured Rat Brain Neurons. Neurochem. J. 15, 8–17 (2021). https://doi.org/10.1134/S1819712421010165
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1819712421010165