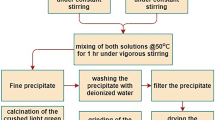
Six variants of direct and reverse coprecipitation of a mixture of salts by an aqueous solution of ammonia or an ammonia buffer solution with pH 9.5 are considered to optimize the technological scheme for the synthesis of zirconium dioxide nanopowders stabilized with 2.5 mol.% yttrium oxide. The influence of the storage time of the mixture of salt solutions before precipitation on the phase and chemical composition, apparent density, and open porosity of sintered specimens was investigated. The best results were obtained using reverse precipitation with an ammonia buffer as precipitant.




Similar content being viewed by others
References
A. A. Boiko and E. N. Poddenezhnyi, “Classification of methods for producing ultradisperse oxide powders (review),” Vestn. GGTU im. P. O. Sukhogo, No. 1, 21 – 28 (2003).
A. O. Zhigachev, Yu. I. Golovin, A. V. Umrikhin, et al., Ceramics Based on Zirconium Dioxide [in Russian], Tekhnosfera, Moscow, 2018, 358 pp.
V. Ya. Shevchenko and S. M. Barinov, Technical Ceramics [in Russian], Nauka, Moscow, 1993, 187 pp.
A. V. Shevchenko, A. K. Ruban, and E. V. Dudnik, “High-tech zirconia-based ceramics,” Ogneupory Tekh. Keram., No. 9, 2 – 8 (2000).
V. N. Antsiferov, S. E. Porozova, and V. B. Kul’met’eva, “Effect of water soluble polymer additives on the phase composition and size of zirconia particles during precipitation from salt solutions,” Glass Phys. Chem., 38(3), 322 – 326 (2012); DOI: https://doi.org/10.1134/S1087659612030029.
I. D. Simonov-Emel’yanov, N. L. Shembel’, E. V. Kostin, and A. D. Kirilin, RU Pat. 2,192,428, IPC C07H19/23, C07F7/00, “Method of synthesis of chelating compounds of zirconium or hafnium with D-fructose,” Nov. 10, 2002; https://findpatent.ru/patent/219/2192428.html.
I. R. Zigan’shin, S. E. Porozova, V. I. Karmanov, et al., “Change in the characteristics of the industrial powder of zirconium oxide and materials based on it by mechanochemical activation,” Russ. J. Non-Ferrous Met., 51, 337 – 341 (2010); DOI: https://doi.org/10.3103/S1067821210040140.
A. Ghosh, A. K. Suri, M. Pandey, et al., “Nanocrystalline zirconia- yttria system — a Raman study,” Mater. Lett., 60(9–10), 1170 – 1173 (2006); DOI: https://doi.org/10.1016/j.matlet.2005.10.102.
B. Liang, C. Ding, H. Liao, et al., “Study on structural evolution of nanostructured 3 mol.% yttria stabilized zirconia coatings during low temperature ageing,” J. Eur. Ceram. Soc., 29(11), 2267 – 2273 (2009); DOI: https://doi.org/10.1016/j.jeurceramsoc.2009.01.002.
S. E. Porozova, V. B. Kul’met’eva, I. R. Zigan’shin, et al., “Comparative characteristics of determination results for monoclinic phase content in zirconium dioxide,” Vopr. Materialoved., No. 1(61), 46 – 52 (2010).
B. Simard, D. M. Rayner, E. Benichou, et al., “Solvation of yttrium with ammonia: an experimental and theoretical study,” J. Phys. Chem. A, 107(43), 9099 – 9104 (2003); DOI: https://doi.org/10.1021/jp035871k.
S. N. Kul’kov, “Structural transformations in nanocrystalline zirconium dioxide,” Poverkhn.: Rentgenovskie, Sinkhrotronnye Neitr. Issled., No. 5, 40 – 43 (2012).
G. Ya. Akimov, V. M. Timchenko, and N. G. Labinskaya, “Effect of the method of stabilization of the tetragonal phase on the mechanical properties of polycrystalline zirconium dioxide,” Fiz. Tverd. Tela, 37(7), 2146 – 2151 (1995).
M. A. Borik, V. T. Bublik, M. Yu. Vilkova, et al., “Structure, phase composition and mechanical properties of ZrO2 crystals partially stabilized with Y2O3,” Mater. Elektron. Tekh., No. 1 (65), 58 – 63 (2014); DOI: https://doi.org/10.17073/1609-3577-2014-1-58-64.
S. V. Gabelkov, R. V. Tarasov, A. G. Mironova, et al., “Behaviour of pore channels and closed pores during sintering of tetragonal zirconia produced from amorphous powder,” Fiz. Tekh. Vys. Davlenii (Donetsk, Ukr.), 21(3), 80 – 93 (2011).
The results were obtained during work on a State Task for the Ministry of Science and Higher Education of the RF (Project No. FSNM-2020-0026).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Novye Ogneupory, No. 11, pp. 38 – 43, November, 2020.
Rights and permissions
About this article
Cite this article
Porozova, S.E., Rogozhnikov, A.G., Shokov, V.O. et al. Optimization of Sol-Gel Conditions for Producing Zirconium Dioxide Nanopowders. Refract Ind Ceram 61, 659–664 (2021). https://doi.org/10.1007/s11148-021-00538-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11148-021-00538-z