Abstract
We investigate the decomposition process of trimethylgallium (TMGa) during GaN metal organic vapor phase epitaxy in detail by using ab inito calculations. We analyze the decomposition rate of TMGa by estimating Gibbs energy of activation including H2 as well as NH3 effects. Our obtained main reaction pathway of TMGa decomposition is as follows: Ga(CH3)3 + 3H2 + NH3 → Ga(CH3)2NH2 + 3H2 + CH4 → Ga(CH3)2H + 2H2 + NH3 +CH4 → GaCH3HNH2 + 2H2 + 2CH4 → GaCH3H2 + H2 + NH3 + 2CH4 → GaH2NH2 + H2 + 3CH4 → GaH3 + NH3 + 3CH4. Our proposed TMGa decomposition pathway can represent the actual epitaxial growth phenomenon by considering neither polymerization reactions nor radical reactions, which are now widely adopted in fluid simulations of crystal growth. Moreover, our proposed pathway is in good agreement with the experiments.
Export citation and abstract BibTeX RIS
1. Introduction
Gallium nitride (GaN) has been attracting great attention as the next-generation semiconductor for power devices. 1,2) GaN is a wide bandgap semiconductor (3.4 eV), which enables high voltage, high speed, and high-temperature operation compared with Si which is the conventional power device material. Therefore, GaN has been actively researched not only for conventional optoelectronic devices but also for future power devices.
Metal-organic vapor phase epitaxy (MOVPE) 3,4) is widely used for the production of GaN. In MOVPE, hydrogen (H2) and nitrogen (N2) carrier gases are used to supply the source gases, trimethyl gallium [TMGa, Ga(CH3)3] and ammonia (NH3), to a substrate with a temperature of 1300 K. However, the detailed TMGa decomposition process has not yet been elucidated. 5–13) Currently, the most commonly employed reaction pathway to model the gas-phase reaction rate of GaN MOVPE has been proposed by Hirako et al. 14) This study states that the main reaction pathway is the reaction with ammonia and amino-related molecules undergo polymerization reactions with each other to form polymers such as 3Ga(CH3)2NH2 → [Ga(CH3)2NH2]2 + Ga(CH3)2NH2 → [Ga(CH3)2NH2]3. Ravasio et al. suggested that in addition to the polymerization reaction, radical reactions by H atoms and NH2 molecules have a significant impact on the main reaction pathway 15) such as Ga(NH2)3 + H → Ga(NH2)2H + NH2. Sekiguchi et al. showed that TMGa repeatedly reacts with H2 and decomposes into GaH 16) by considering the reaction free energies.
In this study, we present a detailed theoretical discussion of the main reaction pathway of the TMGa decomposition by determining the Gibbs energy of activation including H2 in addition to the source gases TMGa and NH3 by ab initio calculations. In this study, we do not consider radicals, since they are very rare chemical species. The content of this paper is as follows. In Sect. 2, we describe the calculation methods. In Sect. 3, calculated results and discussions are shown, and finally, we summarize our findings in Sect. 4.
2. Calculation methods
We used a combination of DFT calculations and transition state theory to analyze chemical reactions during GaN MOVPE growth. The structural optimization of each state, the electronic energy of the ground state, the vibrational frequency calculations and the Gibbs free energy calculations were performed using Gaussian. 16,17) In this study, we used two exchange correlation functionals used in previous studies. The first is B3LYP, 18,19) which is the most commonly used in quantum chemical calculations. This calculation method is thought to be a suitable method for estimating TMGa decomposition reaction. 20–23) The second is the meta-exchange correlation functional M062X. 24) For both calculations, we used 6-311G + (d, p) 25) as the basis set because it allows us to determine the structure and energy of Ga–N with reasonable accuracy. 26)
The Gibbs energy of activation is obtained by calculating the difference between the Gibbs free energies of the initial and transition states, and is an important parameter in considering the reaction process. In order to calculate the Gibbs energy of activation, we obtained the transition structure using the following procedure.
First, we searched for structures close to the transition state using Reaction plus, 27) a software program for which the nudged elastic band method is available. This is a method of searching for a minimum energy path between two different energy minimum states, the initial and the final states. 28,29) Next, we obtained the transition states using Gaussian, 30,31) and then vibrational analysis was performed on each structure, confirming that it has no imaginary frequency at the stable point and only one imaginary frequency in the reaction direction in the transition state. Finally, to confirm that the obtained transition states are the assumed transition states of the reactants and products, intrinsic reaction coordinate calculations were performed. 32,33)
Transition state theory was used to calculate the rate constant, which represents the rate of the reaction. 34) We compared our results with those of other studies, 15,35) in which the same calculation method was used to calculate the rate constants for the reaction of TMGa with NH3, and confirmed that our results adequately represent those of previous studies. In addition, to determine which exchange-correlation functional is appropriate, we compared the Gibbs free energies of several molecules in the reaction path using the coupled cluster singles and doubles (CCSD) method, 36–40) which is a high-precision calculation method. All basis functions in this case, we used aug-c.c.-pVTZ. 41,42)
In this calculation, the frequency is obtained by approximating the energy surface with a quadratic curve, which tends to be larger than the actual frequency. A scaling factor is sometimes used to bring this into better agreement with the experimental frequencies. In this study, the scaling factors of B3LYP[6-311g + (d,p)], B3LYP(aug-c.c.-pVTZ), M062X[6-311g + (d,p)], M062X(aug-c.c.-pVTZ) and CCSD(aug-c.c.-pVTZ) were calculated as 0.9688, 0.9675 and 0.9520, 0.9557, and 0.9558, respectively. 43,44)
It is known that frequencies lower than 150 cm−1 are degenerate to rotational motion. Previous studies have improved the accuracy of chemical reactions by considering lower frequencies as rotators when necessary. 15,35) This low vibrational frequency is mainly associated with the Ga–CH3 bond, and we replaced this vibration with a one-dimensional free rotor.
For the mole fraction calculation, the species conservation equation was solved in time evolution, considering only the chemical reaction term as in Eq. (1).  is the density,
is the density,  is the mass fraction of the chemical species
is the mass fraction of the chemical species  and
and  is the rate of formation of the chemical species
is the rate of formation of the chemical species  and is obtained from the rate constant
and is obtained from the rate constant
3. Results and discussion
3.1. Reaction of TMGa with H2 and NH3 by B3LYP
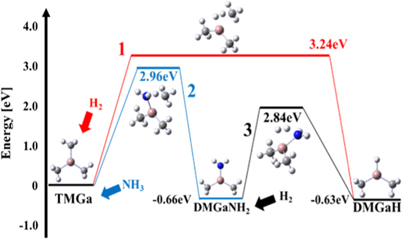
First, to consider the reactions when one methyl group is removed from TMGa, we calculated the Gibbs energy of activation for each chemical reaction. Table I shows the Gibbs energy of activation and rate constants for each chemical reaction at 1300 K. In our previous work, the main reaction pathway is the one in which H2 reacts with TMGa to form dimethyl gallium hydrogen [DMGaH, Ga(CH3)2H]. However, as shown in Fig. 1, the Gibbs energy of activation of TMGa reacting with H2 is 3.24 eV at 1300 K [Reaction Eq. (1); Hereafter R1], whereas the Gibbs energies of activation of TMGa reacting with NH3 first to form an amino group (−NH2) and then reacting with H2 to desorb the amino group are 2.96 eV (R2) and 2.84 eV (R3) respectively, which is smaller than that of reacting with only H2. Calculating the difference in Gibbs energy of activation (3.24 − 2.96 eV = 0.28 eV) using the Eyring equation, it is clear that the reaction with NH3 is faster because the kinetic constant with NH3 is about 12 times larger than the kinetic constant with H2. Therefore, as shown in Fig. 1, the main reaction pathway is not R1, but a combination of R2 and R3.
Table I. Gibbs energy of activation for the TMGa decomposition reaction.
| B3LYP[6-311g + (d,p)] | M062X[6-311g + (d,p)] | ||||
|---|---|---|---|---|---|
| Reaction | Gibbs energy of activation at 1300 K (eV) | Kinetic constant at 1300 K (m3 mol−1 s−1) | Gibbs energy of activation at 1300 K (eV) | Kinetic constant at 1300 K (m3 mol−1 s−1) | |
| 1 | Ga(CH3)3 + H2 → Ga(CH3)2H + CH4 | 3.240 | 0.806 | 3.228 | 0.892 |
| 2 | Ga(CH3)3 + NH3 → Ga(CH3)2NH2 + CH4 | 2.965 | 9.410 | 2.676 | 124.129 |
| 3 | Ga(CH3)2NH2 + H2 → Ga(CH3)2H + NH3 | 2.840 | 28.515 | 2.806 | 38.802 |
| 4 | Ga(CH3)2H + NH3 → Ga(CH3)2NH2 + H2 | 2.811 | 37.160 | 2.556 | 362.661 |
| 5 | Ga(CH3)2NH2 + H2 → GaCH3HNH2 + CH4 | 3.526 | 0.063 | 3.515 | 0.069 |
| 6 | Ga(CH3)2NH2 + NH3 → GaCH3(NH2)2 + CH4 | 3.175 | 1.439 | 2.851 | 25.907 |
| 7 | GaCH3(NH2)2 + H2 → GaCH3HNH2 + NH3 | 3.029 | 5.317 | 2.925 | 13.377 |
| 8 | GaCH3HNH2 + NH3 → GaCH3(NH2)2 + H2 | 2.998 | 6.956 | 2.708 | 93.146 |
| 9 | Ga(CH3)2H + H2 → GaCH3H2 + CH4 | 3.192 | 1.237 | 3.184 | 1.322 |
| 10 | Ga(CH3)2H + NH3 → GaCH3HNH2 + CH4 | 2.942 | 11.530 | 2.665 | 136.413 |
| 11 | GaCH3HNH2 + H2 → GaCH3H2 + NH3 | 2.817 | 35.003 | 2.779 | 49.536 |
| 12 | GaCH3H2 + NH3 → GaCH3HNH2 + H2 | 2.791 | 44.390 | 2.551 | 376.803 |
| 13 | GaCH3(NH2)2 + H2 → GaH(NH2)2 + CH4 | 3.980 | 0.001 | 3.850 | 0.003 |
| 14 | GaCH3(NH2)2 + NH3 → Ga(NH2)3 + CH4 | 3.441 | 0.134 | 3.073 | 3.577 |
| 15 | Ga(NH2)3 + H2 → GaH(NH2)2 + NH3 | 3.124 | 2.270 | 3.342 | 0.323 |
| 16 | GaH(NH2)2 + NH3 → Ga(NH2)3 + H2 | 3.258 | 0.684 | 2.918 | 14.282 |
| 17 | GaCH3HNH2 + H2 → GaH2NH2 + CH4 | 3.516 | 0.068 | 3.501 | 0.079 |
| 18 | GaCH3HNH2 + NH3 → GaH(NH2)2 + CH4 | 3.063 | 3.897 | 2.760 | 58.627 |
| 19 | GaH(NH2)2 + H2 → GaH2NH2 + NH3 | 3.058 | 4.093 | 2.973 | 8.718 |
| 20 | GaH2NH2 + NH3 → GaH(NH2)2 + H2 | 3.024 | 5.526 | 2.755 | 61.339 |
| 21 | GaCH3H2 + H2 → GaH3 + CH4 | 3.157 | 1.687 | 3.161 | 1.628 |
| 22 | GaCH3H2 + NH3 → GaH2NH2 + CH4 | 2.937 | 12.041 | 2.684 | 115.093 |
| 23 | GaH2NH2 + H2 → GaH3 + NH3 | 2.837 | 29.276 | 2.807 | 38.364 |
| 24 | GaH3 + NH3 → GaH2NH2 + H2 | 2.768 | 54.206 | 2.550 | 379.258 |
| 25 | GaH2NH2 + H2 → GaNH2 + 2H2 | 3.610 | 0.030 | 3.917 | 0.002 |
| 26 | GaH3 + H2 → GaH + 2H2 | 3.861 | 0.003 | 4.056 | 0.001 |
| 27 | GaCH3H2 + H2 → GaCH3 + 2H2 | 3.931 | 0.002 | 4.139 | 0.0003 |
| 28 | GaCH3 + H2 → GaH + CH4 | 2.666 | 135.244 | 2.851 | 26.010 |
| 29 | GaCH3 + NH3 → GaNH2 + CH4 | 2.608 | 227.222 | 2.534 | 438.763 |
Fig. 1. (Color online) Energy change in the TMGa decomposition reaction at 1300 K (B3LYP).
Download figure:
Standard image High-resolution imageConsidering the reaction of TMGa as well as the subsequent reactions, 29 chemical reactions are possible, as shown in Table I. The polymerization reactions between NH2 related molecules are not taken into account, and obviously unstable radical molecules and atoms such as NH2 and H are not generated in our considered 29 reactions. We do not consider the reverse reaction in which two hydrogens are desorbed, such as the reaction in which monomethyl gallium (MMGa, GaCH3) is formed (R27; GaCH3H2 + H2 → GaCH3 + 2H2), and the reactions in which GaNH2 and GaH are formed (R25; GaH2NH2 + H2 → GaNH2 + 2H2, R26; GaH3 + H2 →GaH + 2H2). These reactions are called three-body reactions, and in general the probability of three chemical species colliding and reacting is negligibly small.
Next, we considered not only the reaction in which H2 reacts with DMGaNH2 to remove the amino group, but also other reactions together. When H2 and NH3 react with DMGaNH2, there are three possible reactions: R3, R5, and R6. When DMGaNH2 reacts with H2, the Gibbs energy of activation is smaller and the reaction rate is faster when the amino group is removed (R3) than when the methyl group is removed (R5). The results indicate that DMGaNH2 reacts with H2 and changes to DMGaH. This is because the reactivity of H2 with amino groups is higher than that with methyl groups.
In the decomposition of DMGaH, the Gibbs energy of activation when DMGaH reacts with H2 is 3.19 eV (R9), whereas the Gibbs energy of activation when DMGaH first reacts with NH3 to form an amino group and then reacts with H2 to desorb the amino group is 2.94 eV (R10), 2.82 eV (R11), respectively. Similarly, in the decomposition of MMGaH2, the Gibbs energy of activation is lower when reacts with NH3 (R22) and then reacts with H2 (R23) than when H2 reacts directly and the methyl group is removed (R21). In this way, TMGa reacts with NH3 to form an amino group, and then reacts with H2 to desorb the amino group, and by repeating this reaction, the decomposition proceeds. The reactions that produce GaNH2 (R25), GaH (R26), and GaCH3 (R27) are expected to be molecules that are difficult to produce actually because of their high Gibbs energy of activation.
3.2. Reaction of TMGa with H2 and NH3 by M062X
In addition to B3LYP, calculations were also performed for TMGa decomposition reactions with M062X. As shown in Table I, the Gibbs energy of activation of the reaction with H2 did not change much, while the Gibbs energy of activation of the reaction with NH3 became smaller. This implies that the reaction with NH3 is advantageous in M062X. In B3LYP, TMGa reacts with NH3 and then reacts with H2 as a possible main reaction pathway. However, in M062X, the Gibbs energy of activation of the reaction with NH3 (R2) and then with H2 (R3), and the reaction with NH3 in succession (R6) are close. In addition, the reaction of DMGaH with NH3 and back to DMGaNH2 (R4) is likely to occur, so we expect that less DMGaH will be produced and more MMGa(NH2)2 will be produced. In short, In M062X, the sequential pathway in which TMGa reacts with NH3 to form an amino group and then reacts with H2 to desorb the amino group is less likely to occur, indicating that TMGa is more likely to react with NH3 to form an amino group which is in good agreement with the previous report by Ravasio et al. 15)
3.3. Calculation of mole fractions in MOVPE conditions
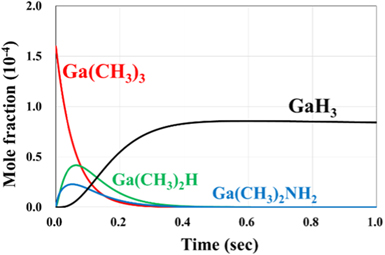
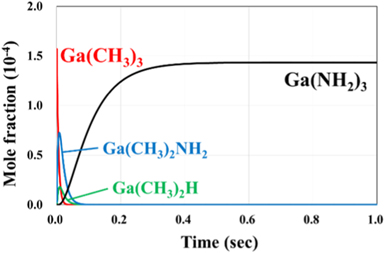
We consider the correspondence between the MOVPE conditions and these reactions for which the reaction rates were determined in the previous section. Figs. 2 and 3 show the time evolution of the mole fraction of each chemical species at 1300 K. In this calculation, we used the rate constants shown in Table I. For the initial partial pressure, the conditions of the MOVPE method were used as a reference, with the total pressure being 1.0 atm, the partial pressures of H2, NH3, and TMGa being 0.50, 0.16,  atm, respectively, and the remainder being N2. For both exchange-correlation functions, the mole fraction is almost constant after 0.5 s, indicating that the value after 1 s is almost the same as the value at equilibrium.
atm, respectively, and the remainder being N2. For both exchange-correlation functions, the mole fraction is almost constant after 0.5 s, indicating that the value after 1 s is almost the same as the value at equilibrium.
Fig. 2. (Color online) Change in mole fraction of each chemical species at 1300 K obtained by B3LYP functional.
Download figure:
Standard image High-resolution imageFig. 3. (Color online) Change in mole fraction of each chemical species at 1300 K obtained by M062X functional.
Download figure:
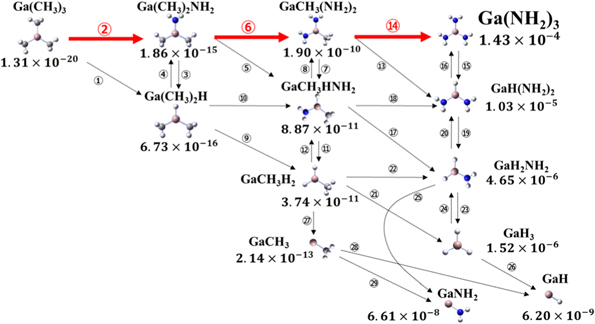
Standard image High-resolution imageFigures 4 and 5 show the mole fractions of each chemical species after 1 s at 1300 K. In B3LYP, as shown in Fig. 4, GaH3, in which three methyl groups are decomposed from TMGa, is the most abundant. The calculated GaH3 ratio is  In M062X, as shown in Fig. 5, Ga(NH2)3, in which three amino groups are formed instead of a methyl group by the reaction with NH3, is the most abundant at
In M062X, as shown in Fig. 5, Ga(NH2)3, in which three amino groups are formed instead of a methyl group by the reaction with NH3, is the most abundant at 
Fig. 4. (Color online) Mole fraction of each chemical species after 1 s at 1300 K and main reaction pathway of TMGa decomposition written in red obtained by B3LYP functional.
Download figure:
Standard image High-resolution imageFig. 5. (Color online) Mole fraction of each chemical species after 1 s at 1300 K and main reaction pathway of TMGa decomposition written in red obtained by M062X functional.
Download figure:
Standard image High-resolution image3.4. Main reaction pathway of TMGa decomposition
From these results, we consider the main reaction pathway of TMGa decomposition for each of the two results. In B3LYP, as shown in Fig. 4, TMGa reacts alternately with NH3 and H2, removing the methyl groups one by one, and decomposes to GaH3. This indicates that the amino group, which is considered to be the cause of the polymerization reaction, is not easily formed and can be represented by a concise chemical reaction equation that does not include a wide variety of polymer molecules. The molecules with methyl groups, which are believed to be the cause of carbon contamination, are very small compared to GaH3. This suggests that decomposing TMGa at a high temperature of about 1300 K for a certain period of time is effective in preventing carbon contamination of the crystal. Furthermore, GaNH2 and GaH, which are considered to be the end products, are less than 1/10 of GaH3. This suggests that GaH3 molecules, which are not considered in the surface reaction in previous studies, must be taken into account.
In M062X, as shown in Fig. 5, TMGa removes the methyl groups one by one by repeated reaction with NH3, and decomposes to Ga(NH2)3. Therefore, this is different from the main reaction pathway estimated for B3LYP. This is consistent with the reaction pathway claimed in previous studies that used M062X to estimate the reaction pathway. 15)
3.5. Comparison between B3LYP and M062X
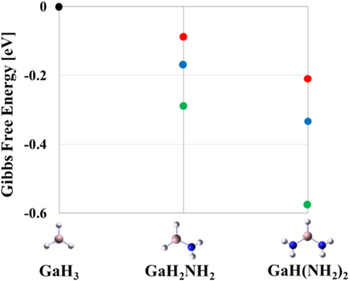
The difference in the energies of the molecules calculated is the key to determining the difference between the final products of B3LYP and M062X. Therefore, in order to discuss which exchange-correlation functional is better for this reaction pathway, we calculated the Gibbs free energy of the molecule by using the high precision CCSD method which includes many electron excitations described by the products of single and double electron excitations. 36–40) The calculations were performed for three molecules, GaH3, GaH2NH2 and GaH(NH2)2. Figure 6 shows the change in the Gibbs free energy with respect to GaH3 at 1300 K. The lower the energy with respect to GaH3, the easier it is for the reaction with NH3 to occur. As a result, the Gibbs free energy calculated using the CCSD method took values between B3LYP and M062X, and GaH3, GaH2NH2, and GaH(NH2)2 were closer to the CCSD values for B3LYP than for M062X. From this result, the main reaction pathway of B3LYP is more representative of the actual phenomenon, and it can be expected that GaH3 is actually more abundant in surface reactions than Ga(NH2)3.
Fig. 6. (Color online) Change in Gibbs free energy at 1300 K with respect to GaH3. Red, blue and green points indicate B3LYP, CCSD, and M062X, respectively. All basis functions we use are aug-cc-pVTZ.
Download figure:
Standard image High-resolution imageMore importantly, the results of time-of-flight mass spectrometry support the occurrence of a reaction with H2 to desorb amino groups by the observation of NH2D molecules under D2 carrier gas conditions. This observation indicates the reaction described as Ga(CH3)2NH2 + D2 → Ga(CH3)2D + NH2D. 45) Therefore, the experiments also indicate that the main reaction pathway obtained by B3LYP is reasonable
4. Conclusions
In this study, to examine the main reaction pathway of the TMGa decomposition process, we calculated and compared the decomposition rate from the Gibbs energy of activation and calculated the mole fraction of each chemical species. As a result, in B3LYP, the decomposition pathway of TMGa is the sequential reaction with NH3 followed by the reaction with H2. In M062X, the decomposition proceeds through repeated reactions with NH3 as in the previous study.
Using the reaction rates we obtained, we calculated the mole fractions of each chemical species at 1300 K. It was found that GaH3 was the most abundant species in B3LYP and Ga(NH2)3 in M062X. When we tested the difference between the two exchange-correlation functions using the high precision CCSD method, it was found that the results of the CCSD method were closer to those of B3LYP. Moreover, the experiment also supports the reaction paths obtained by B3LYP. Therefore, the main reaction pathway of TMGa decomposition is as follows
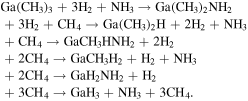
The main reaction pathway obtained in our study shows that when H2 is sufficiently present as a carrier gas, amino groups, which are responsible for the formation of a wide variety of polymers, are difficult to form. Therefore, we were able to reduce the number of chemical reactions considered in conventional fluid simulations. GaH3 molecules must be considered in the surface reaction of GaN MOVPE. Furthermore, it may be effective to decompose TMGa at a high temperature of about 1300 K for a certain period of time to control carbon contamination.
Acknowledgments
We greatly thank Dr. Zheng Ye, Prof. Shugo Nitta, and Prof. Hiroshi Amano for an illuminating discussion from the viewpoints of experiments. This work was supported by the MEXT research programs, "Program for Research and Development of Next-Generation Semiconductors to Realize an Energy-Saving Society under contract No. JPJ005357, "Program for Promoting Research on the Supercomputer Fugaku" (Quantum-Theory-Based Multiscale Simulations toward Development of Next-GenerationEnergy-Saving Semiconductor Devices), and also by the grants-in-aid under contract No. 18H03873. Computations were performed on Fugaku, Riken and on other supercomputers at the Supercomputer Center of ISSP, The University of Tokyo, at the Research Center for Computational Science of the National Institutes of Natural Sciences, at the Information Technology Center of Nagoya University, through the HPCI System Research Project (Project ID: hp200122).