Abstract
This topical review focuses on self-powered electrochemical sensor devices that use wearable biofuel cells (BFCs) that generate electricity from body fluid components, such as glucose in urine and lactate in sweat. The advantages of using BFCs as power sources for wearable health monitoring devices are discussed herein. Recently developed porous carbon materials with controlled interfaces and spaces are also explored for enhancing the output power and stability of BFCs. We describe a printed wearable high-power BFC that uses body fluids as a fuel. This topical review also explains several challenges existing in the development of self-driving health monitoring devices, such as their power output and stability.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Wearable health monitoring devices
For early detection and prevention of diseases, the development of health monitoring devices that do not cause stress or discomfort to the users has been promoted in recent years. As continuous monitoring of health status is an excellent means for improving exercise efficiency and early detection of illness, wearable devices that can easily monitor this information are attracting considerable attention. Owing to the miniaturization and light weight of electronic devices and the rapid increase in internet usage, conventional portable devices are being replaced by wearable devices. Many wearable devices currently available in the market measure physical parameters, such as physical activity, walking distance, respiratory rate, and heart rate, that are closely related to health and exercise efficiency. Furthermore, since 2000, wearable sensors that detect physiological parameters in body fluids such as sweat, saliva, and tears have been developed [1–10]. As these devices can diagnose exercise efficiency and health condition upon wearing them, they are expected to be applied in monitoring health and measuring health-related physical and physiological parameters in daily life and even while exercising. Furthermore, these devices are attracting attention in a wide range of fields, including medicine and public welfare, for example, closed loop integration with drug delivery modules to facilitate out-patient healthcare [11, 12].
Conventional wearable devices are generally powered by lithium-ion batteries or coin-type air batteries. To reduce the sensation of wearing these devices, their sizes need to be reduced. However, when the size of the battery is reduced, the battery capacity decreases, which results in the requirement for frequent charging of a secondary battery or changing of the primary batteries. Therefore, a new power source has to be considered along with safety and manufacturing costs in the downsizing of wearable devices.
Energy harvesting technology that derives electric power from various energies in the environment, such as light, vibration, or heat, is attracting attention [13–16]. Recently, Song et al reported a battery-free wearable sweat sensor powered by a triboelectric nanogenerator [8]. The device contains sodium ion and pH sensors, and Bluetooth for wireless transmission. As wearable devices are required to have a stable and constant power supply all the time, it is desirable to establish an energy harvesting technology that can supply energy sufficiently and sustainably.
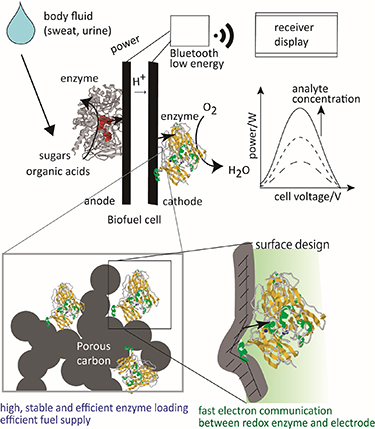
Biofuel cell (BFC) technology has been garnering considerable research interest for energy harvesting in wearable devices (figure 1) [17–22]. A BFC is a power source that uses a biocatalyst as an electrode catalyst. When an enzyme (oxidoreductase) is used as a biocatalyst in a BFC, it is called an enzyme-type BFC; when a microorganism is used, it is called a microbial fuel cell. In this review, unless otherwise specified, BFCs refer to enzyme-type BFCs. Readers are encouraged to refer to some excellent reviews that discuss BFCs in detail [14, 23–26].
Figure 1. Schematic showing the power generation principle of biofuel cells (BFCs).
Download figure:
Standard image High-resolution imageThe use of biocatalysts has the advantages of being capable of generating electricity from organic substances—particularly sugars, organic acids, and alcohols—which are stable, safe, and high-energy-density energy carriers, under mild conditions close to the biological ambient environment (near neutral pH, normal temperature, atmospheric pressure). The advantage of using BFCs as power sources for wearable devices is that they provide high output power density at a relatively low cost. Owing to the high substrate selectivity of an enzyme, BFCs can generate electricity without a separator between the anode and cathode fuels [14, 23–26]. This makes it possible to manufacture a BFC with a simple structure consisting of an enzyme and an electrode. BFCs can be composed of low environmental impact components, and the total cost from manufacturing to disposal is lower than that of conventional portable power generation or power storage devices. As enzymes are proteins, they can be mass-produced through bioengineering, and are not restricted by rare metals in terms of resources.
It is possible to use body fluid as a fuel; therefore, in the future, development of such self-driving wearable devices that generate electricity from the components in body fluids and measure the concentration of these components is highly expected. A self-driving sensor device works according to the following mechanism; for example, glucose in a body fluid reacts with an enzyme in a BFC to convert chemical energy to electric power, which is further used to send a signal from a transmitter. As the output power of the BFC depends on the glucose concentration, the glucose concentration can be determined from the value of the output power of the BFC. A BFC functions as a power source and a sensor. Self-driving devices are being studied globally because they can be the next-generation wearable devices that are light and comfortable to wear [14, 27–29].
In this topical review, we will focus on self-driving sensor devices that use body fluids, especially sweat and urine. Recently reported devices include a contact-lens-type BFC device [30–33] that generates electricity from tears and measures sugar concentration, a mouthpiece-type device [34] that generates electricity from saliva and monitors stress markers, and a patch-type device [35] that monitors lactate concentration in sweat. However, it is necessary to increase the output power to drive the wireless communication device sufficiently without primary or secondary batteries. A hybrid-type BFC that uses a combination of enzymatic anode and abiotic consumed Ag2O|Ag cathode to improve the operational stability and output power has been reported [36].
Our objective is to realize a wearable-type BFC with high power output and high operational stability by developing a specific porous carbon material with a nanointerface designed as an enzyme electrode. In particular, we have investigated devices that generate electricity from the components of body fluid such as glucose [37–39] in urine and lactate [40–42] in sweat. These devices can be manufactured using screen printing, which allows the manufacture of BFCs in large quantities and with low costs on flexible substrates such as paper or plastic film sheets. Paper is a suitable substrate for wearable BFCs, especially for disposable use. Paper is cheap, light, easy to handle, has good storage stability, and can be incinerated and discarded after use. As paper is composed of cellulose or a mixture of cellulose polymers, it has a high affinity toward living organisms. In addition, by further incorporating chemical substances in paper and as a result of the capillary effect, liquid can permeate into hydrophilic fibers without the need for an active pump or an external supply source. Further on in this review we will provide an overview of the BFC followed by a discussion on the recently developed printed porous carbon electrodes and self-driving sensor devices that generate electricity from body fluids.
2. Paper-based BFC and porous carbon materials for improvement of output power
Next, we describe a paper-based BFC fabricated using screen printing. Figure 2 shows the basic design of our paper-based BFC (single cell) [42]. The electro-conductive lead part with holes is printed on a paper substrate. On top of this lead part is printed a porous carbon layer as an enzyme electrode layer (enzyme support). The electrolyte solution containing the fuel can penetrate and be transported to the porous anode and cathode through the holes in the carbon lead layer. For the anode, a hydrophilic binder material that holds the carbon material together is used to improve the penetration of the electrolyte solution. For the cathode, a hydrophobic binder material is used to form a three-phase boundary for efficient oxygen mass-transport from the gas phase.
Figure 2. Schematic showing a paper-based printed BFC.
Download figure:
Standard image High-resolution imageOne of advantages of printed BFCs is that the output power can be modulated according to the application by connecting multiple cells in series if voltage is required, and in parallel if current is required. Therefore, even if the output obtained from one cell is small, it is possible to extract the required power by connecting the cells in series and parallel.
The theoretical electromotive force based on thermodynamics is approximately 1.2 V in the case of glucose/O2 BFC. However, the actual electromotive force of the BFC is approximately 0.6–0.9 V. This is determined by the redox potential difference of the redox species (enzyme or redox mediator) that reacts with the electrode. We can fabricate a BFC system arrayed in series by a printer to boost the operating voltage sufficiently to operate the electric circuit.
The output current can be increased by increasing the electrode surface area. However, for wearable application, a device having a large surface area is not acceptable. Thus, to increase the current density it is necessary to increase the effective surface area for the enzyme and optimize the electrode structure and arrangement considering fuel supply to the enzyme.
In recent years, porous carbon has been attracting increasing attention, and many studies using carbon nanotubes and Ketjen black (KB) have been reported [43–46]. However, the pore-size distribution of the KB-modified electrode ranges from 1 to 100 nm, suggesting that the parts that were smaller than the enzymes were inefficiently used for enzyme loading and electrochemical reactions. Moreover, the enzyme layer that forms on the surface of the KB layer could readily prevent the mass transfer of fuel. A macropore structure enables the rapid mass transfer of enzymes into the porous carbon layer during enzyme electrode fabrication and the smooth mass transfer of fuel during the bioelectrocatalytic reaction.
We previously investigated pore-size-controlled porous carbon materials to improve the BFC output power and its stability. Magnesium-oxide-templated mesoporous carbon (MgOC), which can control the pore size, was used as a porous carbon material for enzyme electrodes [47–58]. The MgOC was fabricated by heating a mixture of carbon precursors, such as polyvinyl alcohol and template MgO, in an inert atmosphere. Thereafter, MgO was removed by washing with dilute H2SO4. Then, MgOC with controlled pore sizes was obtained. The pore size of the MgOC was determined by the size of the MgO crystallite (figure 3). We evaluated cathode electrodes made using MgOC with different sizes [57] and obtained higher current values using porous electrodes with a branched pore structure prepared using MgOC than using electrodes without branched pores [48].
Figure 3. SEM images of an MgO-templated-carbon material with different pore sizes. Reproduced with permission [49]. CC BY 4.0.
Download figure:
Standard image High-resolution image3. Self-driving wearable devices operated using glucose in urine
Many studies have reported on self-driving sensors that monitor glucose in recent years [59–61]. Glucose is an important index for diagnosing diabetes and self-driving sensors are expected to be useful as implantable glucose sensors in the future. Monitoring glucose in urine (urine sugar) is also important. An average of 1.4 l of urine is excreted per day by adults. Urine is composed of water (approximately 91%–96%), inorganic salts, proteins, and hormones. Urine sugar is closely related to blood sugar, and when the blood sugar level rises above the renal threshold, urine sugar can be detected. Sugar begins to appear in urine when the blood glucose level rises above 160–180 mg dl−1. In addition, even if the fasting urine sugar level is within the normal range, 'postprandial hyperglycemia' occurs, in which the postprandial blood glucose level rises along with the urine sugar level. Postprandial hyperglycemia is observed in the early stages of diabetes and often progresses to severe diabetes. It is important to confirm the changes in the urine sugar level while testing for diabetes. Particularly in patients requiring long-term care, it is important to monitor the urine sugar level. However, no self-driving device that generates electricity from urine sugar and monitors the urine sugar level has been developed yet.
Consequently, we have developed a paper-based wearable self-powered glucose biosensor system integrated in a diaper for detecting the urine sugar of diabetic patients [38, 39]. By mounting a paper-based glucose BFC on the diaper, it is possible to generate electricity from urine sugar, detect the timing of urine discharge, and monitor urine sugar. The mental and physical stress on the patient can thereby be reduced. This approach is expected to be useful in homes, senior care facilities, and hospitals. We designed an array structure with cells arranged in a radial pattern and connected in series in order to mount this device on a diaper, be capable of collecting urine, and generate enough output for communication. Anodes and cathodes can be arranged on a planer substrate because it does not require a separator that is typically used in a normal fuel cell.
The disk-shaped BFC developed is a six-series BFC made by printing six electrodes radially on a sheet of paper and pasting them such that the anode and cathode face each other (figure 4). A single cell consists of a bioanode immobilized with GOx and tetrathiafulvalene (TTF) and a biocathode modified with BOD [39]. As six cells are arranged in series, as shown in figure 4, the electromotive force can be set to 3 V without a booster circuit to drive a power-saving wireless transmission circuit, such as Bluetooth low energy, which requires 2.4–2.5 V. The printing technology allows us to easily manufacture an arrayed structure. The circuit-type design is operational even when only a small amount of urine can be supplied to the BFCs. In the prepared disk-shaped BFC, a good linear relationship was obtained between the output and the glucose concentration in the range of 1–25 mM. It was also confirmed that components other than glucose present in urine did not interfere with the output of the BFC. In addition, when glucose solution was supplied, a maximum electromotive force of 3.4 V and a maximum output density of 0.56 mW cm−2 were obtained, and a buzzer and a light-emitting diode connected to the BFC could be driven directly without a booster circuit. This device can be applied to a new diaper BFC capable of detecting urine and monitoring urine sugar levels in future.
Figure 4. (Top) Preparation of a printed BFC for a diaper urine sensor. (Middle) (A) Cell voltage and power curves of a single biofuel cell as a function of current density (using 0.1 M glucose in 1 M phosphate buffer solution). (B) Dependence of the power-current curve on the concentration of glucose. (Bottom) Cell voltage and power curves of the biofuel cell array as a function of current density. Reproduced from [39]. © IOP Publishing Ltd. CC BY 3.0.
Download figure:
Standard image High-resolution imageVery recently, we reported a paper-based printed BFC preloaded with glucose and phosphate buffer salts that can generate electricity when supplied with water only [62]. A new four-series/four-parallel structured paper-substrate BFC (figure 5) preloaded with glucose and electrolytes produced an output of approximately 0.84 mW when water was added. This is 90% of that obtained by supplying a phosphate buffer containing glucose as the electrolyte. The open-circuit voltage was 2.1 V, and an LED could be powered by simply supplying water to the cell without using a booster circuit. This BFC has potential application to the diaper BFC-type urine-timing sensor for non-diabetic patients.
Download figure:
Standard image High-resolution image4. Self-driving wearable device operated using lactate in sweat
Efforts to link advanced engineering and information science with conventional sports science are increasing. Evaluating performance during exercise or sports in real time will make it possible to provide feedback on training effects to the athletes, prevent overtraining, and propose effective recovery methods. Wearable sensors are already being used for non-invasive monitoring of vital signs such as pulse rate. However, non-invasive monitoring of lactate and glucose levels in sweat is important to monitor and manage other health conditions of athletes. At present, there is no such non-invasive real-time monitoring device for lactate and glucose levels during exercise, instead blood samples are taken during exercise. The blood sampling makes it difficult to obtain accurate values during exercise because of the strong mental and physical stress on the athlete. Additionally, the time delay to obtain the output is not negligible because some time is required between sample collection and sample analysis.
Sweat contains water, electrolytes (chloride, sodium, potassium), metals (iron, magnesium, zinc), and metabolites (lactate, glucose, urea, creatinine) in abundance [63]. The concentrations of these biomarkers in sweat provide important information on health and physical performance. Furthermore, the information on the concentrations of these biomarkers can be used for various diagnoses in evaluating sports performance. Lactate is a very promising power source for such sensing processes [64, 65] because of its high concentration in the sweat (5–40 mM) of healthy subjects. Conversely, the glucose concentration in sweat is too low to power a BFC. Thus, a lactate/O2 BFC can be a promising candidate as a wearable power source. As mentioned above, the BFC generates electricity by the reaction of the substrate of the enzyme immobilized on the anode. The output of the BFC increases with the lactate concentration and reaches the maximum value, unless the cathode performance limits the cell performance. That is, the lactate concentration can be determined by detecting the output power of the BFC. For these reasons, various lactate-fueled self-driving biosensors are being researched globally. For example, Wang et al developed a tattoo-type BFC with low wearing sensation that can generate approximately 40 μW cm−2 of electricity using the lactate in sweat [66]. Bandodkar et al fabricated electrodes using screen printing and carbon nanotubes and prepared a flexible lactate BFC with high power density [67]. Villalpando et al developed a patch-type enzyme BFC in which a wireless transmission device monitors the output of the BFC during exercise [68, 69]. Russell et al reported a contact-lens-type BFC that uses lactate in tears as a fuel through a buckypaper electrode on a silicon soft contact lens and succeeded in obtaining an output of approximately 8 μW cm–2 [70]. However, to put the lactate BFC into practical use as a power source for wearable devices including a wireless transmitter, improvements in output, current density, and voltage are still necessary.
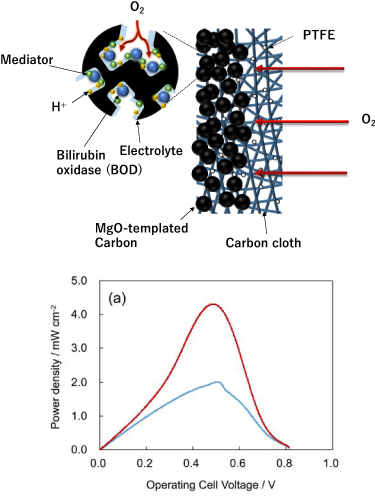
As described above, immobilization of enzymes and mediators and highly efficient substance transport on substrates are important for increasing the output of enzyme-type BFCs and their operational stability. In particular, for the BFC operating on the skin, the release of enzyme and mediator should be prevented. We constructed lactate BFCs using the aforementioned MgOC material to achieve high stability and output power for BFCs [40]. A high output cannot be obtained unless the substrate can be efficiently supplied to the enzyme. At the anode, the substrate is supplied from the electrolyte solution. In contrast, at the cathode, normally dissolved oxygen is used as a fuel, but the dissolved oxygen concentration is as low as 0.25 mM. Thus, it is important to develop a gas diffusion electrode that uses atmospheric oxygen as a fuel for highly efficient oxygen reduction to improve the output of BFCs [71]. Our lactate/O2 BFC was prepared integrated with a 1-methoxy PMS-LOx-modified anode and BOD-modified air-diffusion biocathode, which is made by coating a porous carbon ink containing 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), which acts as an anionic surfactant and a redox mediator, onto the water-repellent carbon cloth, treated with polytetrafluoroethylene, to form a gas diffusion electrode (figure 6). The maximum output densities were obtained with an open-circuit voltage of 0.81 V and 2.0 mW cm−2 in the atmosphere and 4.3 mW cm−2 under oxygen flow. Even at a low lactate concentration of 20 mM, the output was over 1 mW.
Figure 6. (Top) Schematic showing an air-diffusion type biocathode. (Bottom) Cell voltage–power density curves of the lactate/O2 BFC in 1.5 M PBS containing 300 mM lactate operated under air (blue curve) and O2 (red curve). Reproduced from [62]. © IOP Publishing Ltd. CC BY 3.0.
Download figure:
Standard image High-resolution imageWe are currently developing a self-driving wearable sensor device equipped with a printed 6 × 6 array-type lactate BFC, an array comprising six cells in series and six cells in parallel [72]. The BFC with an efficient fuel supply system affords an open-circuit voltage of approximately 3.4 V and a maximum output of 4.3 mW, which is sufficient for driving wireless communication tools, such as Bluetooth low energy devices, for wireless transmission without requiring a booster circuit. The output power density increases proportionally with the concentration of lactate in the range of 2.5–25 mM, covering the normal lactate concentration range. This array-type BFC is expected to be used as a self-driven wearable monitoring tool for monitoring the physiological conditions of athletes and preventing heat strokes.
5. Perspective
Self-driving devices using body fluids are light, compact, flexible, inexpensive, environment friendly, and safe. For the practical application of self-driving devices equipped with BFCs that use body fluids as fuel, the top priority is to improve output and stability (life and durability). The output is determined by slow fuel supply rate or enzyme electrode reaction rate. The fuel supply rate is determined by the BFC configuration, electrode structure, fuel concentration, and solution viscosity. In the presence of sufficient fuel, the performance of the enzyme electrode is determined by the amount and activity of the enzyme on the electrode. Therefore, to improve the output current, the BFC requires an electrode that can achieve rapid fuel transport and an enzyme having high electrode catalytic activity (screening, protein engineering modification, etc). It is important to develop nanostructured interfaces with porous materials that effectively increase the amount of electrochemically active enzyme per geometric surface. Enzymes lose their activities owing to structural changes and degradation caused by pH and temperature changes. Factors such as aggregation and desorption of enzymes from the electrodes also affect the stability of enzyme electrodes. When an electron transfer mediator is used, its detachment from the electrode surface contributes toward a decrease in the output. Therefore, to improve the durability of wearable BFCs, it is important to develop a highly durable enzyme, support the enzyme using a three-dimensional structure, improve the operating environment such as the type of electrolyte used [73, 74], and modify the electrode surface when using a mediator.
In a glucose anode, GOx has been the standard bioelectrocatalyst for glucose oxidation in BFC anodes owing to its high thermal stability and superior selectivity for glucose. Flavin adenine dinucleotide (FAD)-dependent glucose dehydrogenase (FADGDH) has emerged as a powerful alternative for glucose-oxidizing bioanodes owing to high turnover rates and substrate selectivity, good stability, and oxygen insensitivity [75, 76]. Unlike GOx, FADGDH does not use oxygen as an electron acceptor, thus avoiding enzymatic production of hydrogen peroxide. Hydrogen peroxide damages enzyme electrodes, thereby reducing their lifetimes [77]. For lactate anodes, LOx is the most widely used enzyme, but this enzyme uses oxygen as a natural electron acceptor. Protein-engineered lactate dehydrogenase is developed for electrochemical applications [78]. It is expected that constructing a direct electron transport chain without using a soluble mediator will also lead to improved stability. BOD can communicate directly without a redox mediator, but GOx and fungal FADGDH require redox mediators. Protein-engineered fungal or bacterial FADGDHs can be used as direct-electron-transfer (DET)-type electrocatalysts instead of fungal FADGDHs, and recent advances in nanostructured electrode materials allow DET in fungal FADGDHs [79, 80].
For mediator modification, poly(glycidyl methacrylate) is modified on the MgOC surface through electron beam graft polymerization [81–83]. A redox mediator can be immobilized by covalent bonding between the amino group and pendant glycidyl group. The glycidyl group can immobilize enzymes as well. The electrochemical modification of phenothiazine-type mediators gives robust covalently modified electrodes. Cathodic electrografting of phenothiazine diazonium and anodic grafting of phenothiazine are performed for bioelectrocatalytic glucose oxidation with fungal FADGDH [84, 85]. The electrografted polymer-type phenothiazine surface assemblies exhibit superior mediated bioelectrocatalytic properties compared to the adsorbed phenothiazine electrodes. Chemical polymerization of phenothiazine and methylene green on a 3D nanocarbon material resulted in high electrode stability for over a year during bioanode electrocatalytic activity in glucose oxidation catalyzed by NADP-type GDH [86].
In recent years, owing to recent remarkable technological innovations, BFCs have been producing a higher output power density and becoming smaller and more flexible. To achieve performance levels suitable for practical use, high-performance enzymes are being developed utilizing bioengineering methods, and advancements in electrochemistry have led to the development of BFCs. It is expected that innovative performance improvements with synergistic effects and lower power consumption of various electronic devices will produce new healthcare, information communication, and IoT devices in future.
Acknowledgments
This work was partially supported by JST-ASTEP Grant No. JPMJTS1513 (IS, ST), JSPS Grant No. 17H02162(IS), 18H01719(ST), the Private University Research Branding Project (2017–2019) from the Ministry of Education, Culture, Sports, Science and Technology (IS), and the Tokyo University of Science Grant for the President's Research Promotion (IS).