Abstract
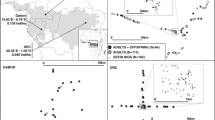
Seed and pollen dispersal are important for defining sustainable forest management practices. By reducing population density, selective logging could affect not only the seed production of timber species but also the selfing rate and the patterns of seed and pollen rains. To assess these risks, we characterized seed and pollen dispersal patterns and the fine-scale spatial genetic structure (FSGS) of Pericopsis elata, a gregarious, wind-dispersed legume tree which is highly logged in Central Africa and threatened by overexploitation. Eleven microsatellite markers were used to genotype 189 adults and 664 seedlings in a 4 km2 plot in the Democratic Republic of Congo (DRC). According to the neighbourhood model, seed dispersal was extremely leptokurtic, with 80% of seeds dispersal distances <75 m, 15% >500 m. Pollen dispersal was locally more extensive (median distance 260 m), but pollen immigration was not detected, and the selfing rate (54%) appeared particularly high compared to other tropical tree species. Limited gene dispersal resulted in remarkably high FSGS (Sp = 0.072). A decay of inbreeding with age also suggests that the species is prone to inbreeding depression. The reproductive success of trees was positively related to their diameter at breast height (dbh), with half of the progeny mothered by trees with dbh > 97 cm and fathered by trees with dbh > 119 cm. Our study highlights that (1) seed sources must be diversified for plantation or population reinforcement to limit consanguinity, and (2) the legal minimum cutting diameter in DRC (60 cm) should be increased to maintain enough post-logging reproductive potential.





Similar content being viewed by others
Data availability
The genotypes, diameters, and spatial coordinates of the trees within the 400 ha plot are available in the TreeGenes Database under code TGDR368 (https://treegenesdb.org/completed-submission/TGDR368).
References
Ampofo ST, Lawson GW (1972) Growth of seedlings of Afrormosia elata harms in relation to light intensity. The Journal of Applied Ecology 9:301. https://doi.org/10.2307/2402063
André T, Lemes MR, Gribel R (2008) Post-logging loss of genetic diversity in a mahogany (Swietenia macrophylla King, Meliaceae) population in Brazilian Amazonia. For Ecol Manag 255:340–345. https://doi.org/10.1016/J.FORECO.2007.09.055
Arnhem E, Dupain J, Drubbel RV et al (2008) Selective logging, habitat quality and home range use by sympatric gorillas and chimpanzees: a case study from an active logging concession in Southeast Cameroon. Folia primatologica; international journal of primatology 79:1–14. https://doi.org/10.1159/000107664
Bittencourt JVM, Sebbenn AM (2007) Patterns of pollen and seed dispersal in a small, fragmented population of the wind-pollinated tree Araucaria angustifolia in southern Brazil. Heredity 99:580–591. https://doi.org/10.1038/sj.hdy.6801019
Bittencourt JVM, Sebbenn AM (2008) Pollen movement within a continuous forest of wind-pollinated Araucaria angustifolia, inferred from paternity and TwoGener analysis. Conserv Genet 9:855–868. https://doi.org/10.1007/s10592-007-9411-2
Bizoux JP, Daînou K, Bourland N et al (2009) Spatial genetic structure in Milicia excelsa (Moraceae) indicates extensive gene dispersal in a low-density wind-pollinated tropical tree. Mol Ecol 18:4398–4408. https://doi.org/10.1111/j.1365-294X.2009.04365.x
Born C, Hardy OJ, Chevallier MH et al (2008) Small-scale spatial genetic structure in the Central African rainforest tree species Aucoumea klaineana: a stepwise approach to infer the impact of limited gene dispersal, population history and habitat fragmentation. Mol Ecol 17:2041–2050. https://doi.org/10.1111/j.1365-294X.2007.03685.x
Bourland N, Kouadio Y, Lejeune P et al (2012a) Ecology of Pericopsis elata (Fabaceae), an endangered timber species in southeastern Cameroon. Biotropica 44(6):840–847
Bourland N, Kouadio YL, Fétéké F et al (2012b) Ecology and management of Pericopsis elata (Harms) Meeuwen (Fabaceae) populations: a review. Biotechnol Agron Soc Environ 16:486–498
Bourland N, Cerisier F, Daïnou K, Smith A, Hubau W, Beeckman H, Brostaux Y, Fayolle A, Biwolé A, Fétéké F, Gillet JF, Morin-Rivat J, Lejeune P, Tiba E, van Acker J, Doucet JL (2015) How tightly linked are Pericopsis elata (Fabaceae) patches to anthropogenic disturbances in Southeastern Cameroon? Forests 6:293–310. https://doi.org/10.3390/f6020293
Boyemba Bosela F (2011) Ecologie de Pericopsis elata (Harms) Van Meeuwen (Fabaceae), arbre de forêt tropicale africaine à répartition agrégée
Chybicki IJ (2018) NMπ—improved re-implementation of NM+, a software for estimating gene dispersal and mating patterns. Mol Ecol Resour 18(1):159–168
Chybicki IJ, Burczyk J (2009) Simultaneous estimation of null alleles and inbreeding coefficients. J Hered 100:106–113. https://doi.org/10.1093/jhered/esn088
Chybicki IJ, Burczyk J (2010) NM+: Software implementing parentage-based models for estimating gene dispersal and mating patterns in plants. Mol Ecol Resour 10:1071–1075. https://doi.org/10.1111/j.1755-0998.2010.02849.x
Clark C, Poulsen J, … RM-C (2009) Logging concessions can extend the conservation estate for Central African tropical forests. Wiley Online Library
Cloutier D, Hardy OJ, Caron H, Ciampi AY, Degen B, Kanashiro M, Schoen DJ (2007) Low inbreeding and high pollen dispersal distances in populations of two Amazonian forest tree species. Biotropica 39:406–415. https://doi.org/10.1111/j.1744-7429.2007.00266.x
da Silva Carneiro F, Magno Sebbenn A, Kanashiro M, Degen B (2007) Low interannual variation of mating system and gene flow of Symphonia globulifera in the Brazilian Amazon. Biotropica 39:628–636. https://doi.org/10.1111/j.1744-7429.2007.00314.x
David P, Pujol B, Viard F et al (2007) Reliable selfing rate estimates from imperfect population genetic data. Mol Ecol 16:2474–2487. https://doi.org/10.1111/j.1365-294X.2007.03330.x
de Lacerda AEB, Kanashiro M, Sebbenn AM (2008) Effects of reduced impact logging on genetic diversity and spatial genetic structure of a Hymenaea courbaril population in the Brazilian Amazon Forest. For Ecol Manag 255(3-4):1034–1043
de Wasseige C, de Marcken P, Bayol N, et al (2012) The forests of the Congo Basin: state of the forest 2010. p 276. https://doi.org/10.2788/47210
De Wasseige C, Flynn J, Louppe D, Hiol Hiol F, & Mayaux P (2014) The forests of the Congo Basin-state of the forest 2013. Weyrich
Dick CW, Hardy OJ, Jones FA, Petit RJ (2008) Spatial scales of pollen and seed-mediated gene flow in tropical rain forest trees. Trop Plant Biol 1:20–33. https://doi.org/10.1007/s12042-007-9006-6
Dickson B, Mathew P, Mickleburgh S, et al (2005) An assessment of the conservation status, management and regulation of the trade in Pericopsis elata. cites.org. 10.1
Doligez A, Joly HI (1997) Genetic diversity and spatial structure within a natural stand of a tropical forest tree species, Carapa procera (Meliaceae), in French Guiana. Heredity 79:72–82. https://doi.org/10.1038/hdy.1997.124
Doucet J, Réserves YK-P et (2007) undefined Le moabi, une espèce «phare» de l’exploitation forestière en Afrique centrale. pallisco-cifm.com
Doucet JL, Daïnou K, Ligot G, Ouédraogo DY, Bourland N, Ward SE, Tekam P, Lagoute P, Fayolle A (2016) Enrichment of Central African logged forests with high-value tree species: testing a new approach to regenerating degraded forests. International Journal of Biodiversity Science, Ecosystem Services and Management 12:83–95. https://doi.org/10.1080/21513732.2016.1168868
Duminil J, Hardy OJ, Petit RJ (2009) Plant traits correlated with generation time directly affect inbreeding depression and mating system and indirectly genetic structure. BMC Evol Biol 9:177. https://doi.org/10.1186/1471-2148-9-177
Duminil J, Mendene Abessolo DT, Ndiade Bourobou D, Doucet JL, Loo J, Hardy OJ (2016a) High selfing rate, limited pollen dispersal and inbreeding depression in the emblematic African rain forest tree Baillonella toxisperma – management implications. For Ecol Manag 379:20–29. https://doi.org/10.1016/j.foreco.2016.08.003
Duminil J, Daïnou K, Kaviriri DK, Gillet P, Loo J, Doucet JL, Hardy OJ (2016b) Relationships between population density, fine-scale genetic structure, mating system and pollen dispersal in a timber tree from African rainforests. Heredity 116:295–303. https://doi.org/10.1038/hdy.2015.101
Dutech C, Seiter J, Petronelli P, Joly HI, Jarne P (2002) Evidence of low gene flow in a neotropical clustered tree species in two rainforest stands of French Guiana. Mol Ecol 11:725–738. https://doi.org/10.1046/j.1365-294X.2002.01475.x
Eduardo A, De Lacerda B, Kanashiro M, Sebbenn AM (2008) Long-pollen movement and deviation of random mating in a low-density continuous population of a tropical tree Hymenaea courbaril in the Brazilian Amazon. Biotropica 40:462–470. https://doi.org/10.1111/j.1744-7429.2008.00402.x
Ewédjè EBK, Ahanchédé A, Hardy OJ (2017) Breeding system, gene dispersal and small-scale spatial genetic structure of a threatened food tree species, Pentadesma butyracea (Clusiaceae) in Benin. Conserv Genet 18(4):799–811
Fayolle A, Ouédraogo DY, Ligot G, Daïnou K, Bourland N, Tekam P, Doucet JL (2015) Differential performance between two timber species in forest logging gaps and in plantations in Central Africa. Forests 6:380–394. https://doi.org/10.3390/f6020380
Fenster CB, Vekemans X, Hardy OJ (2003) Quantifying gene flow from spatial genetic structure data in a metapopulation of Chamaecrista fasciculata (Leguminosae). Evolution 57:995–1007. https://doi.org/10.1111/j.0014-3820.2003.tb00311.x
Gerber S, Latouche-Hallé C, Lourmas M et al (2003) Mesure directe des flux de gènes en forêt. Les Actes du BRG 4:349–368
Hall JS, Harris DJ, Medjibe V, Ashton PMS (2003) The effects of selective logging on forest structure and tree species composition in a Central African forest: implications for management of conservation areas. For Ecol Manag 183:249–264. https://doi.org/10.1016/S0378-1127(03)00107-5
Hardy OJ, Vekemans X (2002) spagedi: a versatile computer program to analyse spatial genetic structure at the individual or population levels. Mol Ecol Notes 2:618–620. https://doi.org/10.1046/j.1471-8286.2002.00305.x
Hardy OJ, Maggia L, Bandou E, Caron H, Chevallier MH, Doligez A et al (2006) Fine-scale genetic structure and gene dispersal inferences in 10 Neotropical tree species. Mol Ecol 15:559–571
Hardy OJ, Delaide B, Hainaut H et al (2019) Seed and pollen dispersal distances in two African legume timber trees and their reproductive potential under selective logging. Mol Ecol 28(12):3119–3134. https://doi.org/10.1111/mec.15138
Haurez B, Petre C, Doucet J (2013) Impacts of logging and hunting on western lowland gorilla (Gorilla gorilla gorilla ) populations and consequences for forest regeneration. A review Base 17:364–372
Hufford KM, Hamrick JL (2003) Viability selection at three early life stages of the tropical tree, Platypodium elegans (Fabaceae, Papilionoideae). Evolution 57:518–526. https://doi.org/10.1111/j.0014-3820.2003.tb01543.x
Ismail SA, Kokko H (2020) An analysis of mating biases in trees. Mol Ecol 29:184–198. https://doi.org/10.1111/mec.15312
Jones FA, Chen J, Weng G-J, Hubbell SP (2005) A genetic evaluation of seed dispersal in the neotropical tree Jacaranda copaia (Bignoniaceae). Am Nat 166:543–555. https://doi.org/10.1086/491661
Kettle CJ, Hollingsworth PM, Burslem DFRP, Maycock CR, Khoo E, Ghazoul J (2011) Determinants of fine-scale spatial genetic structure in three co-occurring rain forest canopy trees in Borneo. Perspectives in Plant Ecology, Evolution and Systematics 13:47–56. https://doi.org/10.1016/j.ppees.2010.11.002
Kremer A, Ronce O, Robledo-Arnuncio JJ, Guillaume F, Bohrer G, Nathan R, Bridle JR, Gomulkiewicz R, Klein EK, Ritland K, Kuparinen A, Gerber S, Schueler S (2012) Long-distance gene flow and adaptation of forest trees to rapid climate change. Ecol Lett 15:378–392
Lee S (2000) Mating system parameters of Dryobalanops aromatica Gaertn. f. (Dipterocarpaceae) in three different forest types and a seed orchard. Heredity 85:338–345. https://doi.org/10.1046/j.1365-2540.2000.00761.x
Loiselle BA, Sork VL, Nason J, Graham C (1995) Spatial genetic structure of a tropical understory shrub, Psychotria officinalis (Rubiaceae). Am J Bot 82:1420–1425. https://doi.org/10.1002/j.1537-2197.1995.tb12679.x
Lomba B (2011) Systèmes d’agrégation et structures diamétriques en fonction des tempéraments de quelques essences dans les dispositifs permanents de Yoko et Biaro
Lourmas M, Kjellberg F, Dessard H, Joly HI, Chevallier MH (2007) Reduced density due to logging and its consequences on mating system and pollen flow in the African mahogany Entandrophragma cylindricum. Heredity 99:151–160. https://doi.org/10.1038/sj.hdy.6800976
Micheneau C, Dauby G, Bourland N, Doucet JL, Hardy OJ (2011) Development and characterization of microsatellite loci in Pericopsis elata (Fabaceae) using a cost-efficient approach. Am J Bot 98(10):e268–e270. https://doi.org/10.3732/ajb.1100070
Monthe FK, Hardy OJ, Doucet JL, Loo J, Duminil J (2017) Extensive seed and pollen dispersal and assortative mating in the rain forest tree Entandrophragma cylindricum (Meliaceae) inferred from indirect and direct analyses. Mol Ecol 26:5279–5291. https://doi.org/10.1111/mec.14241
Muller-Landau HC, Wright SJ, Calderón O, Condit R, Hubbell SP (2008) Interspecific variation in primary seed dispersal in a tropical forest. J Ecol 96:653–667. https://doi.org/10.1111/j.1365-2745.2008.01399.x
Nanson A (2004) Génétique et amélioration des arbres forestiers
Nathan R, Muller-Landau HC (2000) Spatial patterns of seed dispersal, their determinants and consequences for recruitment
Ndiade-Bourobou D, Hardy OJ, Favreau B et al (2010) Long-distance seed and pollen dispersal inferred from spatial genetic structure in the very low-density rainforest tree, Baillonella toxisperma Pierre, in Central Africa. Mol Ecol 19:4949–4962. https://doi.org/10.1111/j.1365-294X.2010.04864.x
Nei M (1978) Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89(3):583-590
Nielsen R, Tarpy DR, Reeve HK (2003) Estimating effective paternity number in social insects and the effective number of alleles in a population. Mol Ecol 12:3157–3164. https://doi.org/10.1046/j.1365-294X.2003.01994.x
Ouédraogo D, Fayolle A, Daïnou K, Demaret C (2014) Enrichment of logging gaps with a high conservation value species (Pericopsis elata) in a Central African Moist Forest. Forests 5:3031–3047
Porcher E, Lande R (2005) The evolution of self-fertilization and inbreeding depression under pollen discounting and pollen limitation. J Evol Biol 18(3):497–508. https://doi.org/10.1111/j.1420-9101.2005.00905.x
Robledo-Arnuncio JJ (2011) Wind pollination over mesoscale distances: an investigation with Scots pine. New Phytol 190:222–233. https://doi.org/10.1111/j.1469-8137.2010.03588.x
Schroeder JW, Tran HT, Dick CW (2014) Fine scale spatial genetic structure in Pouteria reticulata (Engl.) Eyma (Sapotaceae), a dioecious, vertebrate dispersed tropical rain forest tree species. Global Ecology and Conservation 1:43–49
Sebbenn A, Carvalho A, Freitas M, Moraes B, Gaino A, Da Silva JM et al (2011) Low levels of realized seed and pollen gene flow and strong spatial genetic structure in a small, isolated and fragmented population of the tropical tree Copaifera langsdorffii Desf. Heredity 106(1):134–145
Toussaint L, Wilczek R, Gillett J, et al (1953) Flore du Congo Belge et du Ruanda-Urundi: Papilionaceae (Sophoreae, Genisteae, Trifolieae et Loteae)
Umunay PM, Covey KR, Makana JR, Gregoire TG (2017) Effect of light, fire and weed control on establishment of Pericopsis elata Harms regeneration. New For 48:735–752. https://doi.org/10.1007/s11056-017-9594-4
Veenendaal EM, Swaine MD, Lecha RT, Walsh MF, Abebrese IK, Owusu-Afriyie K (1996) Responses of West African Forest tree seedlings to irradiance and soil fertility. Funct Ecol 10:501. https://doi.org/10.2307/2389943
Vekemans X, Hardy OJ (2004) New insights from fine-scale spatial genetic structure analyses in plant populations. Mol Ecol 13:921–935. https://doi.org/10.1046/j.1365-294X.2004.02076.x
Wickneswari R, Ho W, Lee K, et al (2004) Impact of disturbance on population and genetic structure of tropical forest trees. academia.edu
Acknowledgements
We are grateful to the entire EBE team and their constructive reviews and comments that have helped us to improve our manuscript. We also thank the Compagnie Forestière et de Transformation (CFT), and especially the General Director K. Ammacha, for hosting us during our fieldwork, as well as all the technicians of the Ecology and Forest Management Laboratory (LECAFOR)/UNIKIS for their help during the collection of samples. We thank two anonymous reviewers for their constructive comments and Grace Ridder for checking the text.
Code availability (software application or custom code)
Not relevant here
Funding
This research was supported by the “FCCC” and “FORETS” project, funded by the European Union, and implemented at the University of Kisangani by CIFOR in collaboration with Resources and Synergies Development, through the award of a doctoral fellowship to DMAA, which allowed us to work successfully at the Laboratory of Evolutionary Biology and Ecology “EBE”. Molecular analyses were funded by the F.R.S-FNRS WISD grant X.3040.17 (project AFRITIMB). This research was also backed by the N3-GeForCo Project, funded by the Belgian Cooperation and implemented by the Royal Museum for Central Africa.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by J.P. Jaramillo-Correa
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Angbonda, D..M.A., Monthe, F.K., Bourland, N. et al. Seed and pollen dispersal and fine-scale spatial genetic structure of a threatened tree species: Pericopsis elata (HARMS) Meeuwen (Fabaceae). Tree Genetics & Genomes 17, 27 (2021). https://doi.org/10.1007/s11295-021-01509-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11295-021-01509-8