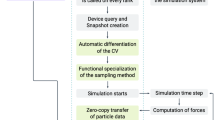
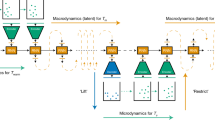
Abstract
Multiscale simulations are a well-accepted way to bridge the length and time scales required for scientific studies with the solution accuracy achievable through available computational resources. Traditional approaches either solve a coarse model with selective refinement or coerce a detailed model into faster sampling, both of which have limitations. Here, we present a paradigm of adaptive, multiscale simulations that couple different scales using a dynamic-importance sampling approach. Our method uses machine learning to dynamically and exhaustively sample the phase space explored by a macro model using microscale simulations and enables an automatic feedback from the micro to the macro scale, leading to a self-healing multiscale simulation. As a result, our approach delivers macro length and time scales, but with the effective precision of the micro scale. Our approach is arbitrarily scalable as well as transferable to many different types of simulations. Our method made possible a multiscale scientific campaign of unprecedented scale to understand the interactions of RAS proteins with a plasma membrane in the context of cancer research running over several days on Sierra, which is currently the second-most-powerful supercomputer in the world.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Sample data for DynIm is also made available along with the code repository. The data related to the multiscale simulation described in the paper will be made available upon reasonable request; the size of all raw data is hundreds of terabytes. For more information, please see details of the simulation14
Code availability
The framework for DynIm has been released open source under the MIT license: https://github.com/LLNL/dynim.
References
Ingram, G., Cameron, I. & Hangos, K. Classification and analysis of integrating frameworks in multiscale modelling. Chem. Eng. Sci. 59, 2171–2187 (2004).
Weinan, E. Principles of Multiscale Modeling (Cambridge Univ. Press, 2011).
Hoekstra, A., Chopard, B. & Coveney, P. Multiscale modelling and simulation: a position paper. Phil. Trans. R. Soc. A 372, 20130377 (2014).
Chopard, B., Borgdorff, J. & Hoekstra, A. A framework for multi-scale modelling. Phil. Trans. R. Soc. A 372, 20130378 (2014).
Geweke, J. Bayesian inference in econometric models using Monte Carlo integration. Econometrica 57, 1317–39 (1989).
MacKay, D. J. C. Information Theory, Inference, and Learning Algorithms (Cambridge Univ. Press, 2003).
Liang, F. Dynamically weighted importance sampling in Monte Carlo computation. J. Am. Stat. Assoc. 97, 807–821 (2002).
Liang, F. & Cheon, S. Monte Carlo dynamically weighted importance sampling for spatial models with intractable normalizing constants. J. Phys. Conf. Ser. 197, 012004 (2009).
Joubert, D. J. & Marwala, T. Monte Carlo dynamically weighted importance sampling for finite element model updating. In Topics in Modal Analysis and Testing (ed. Mains, M.) Vol. 10, 303−312 (Springer, 2016).
Katharopoulos, A. & Fleuret, F. Not all samples are created equal: deep learning with importance sampling. In Proc. 35th Int. Conf. on Machine Learning (eds Dy, J. & Krause, A.) Vol. 80, 2525−2534 (Proceedings of Machine Learning Research, 2018). http://proceedings.mlr.press/v80/katharopoulos18a.html"
Johnson, T. B. & Guestrin, C. Training deep models faster with robust, approximate importance sampling. In Advances in Neural Information Processing Systems (eds Bengio, S. et al.) Vol. 31, 7265−7275 (Curran Associates, 2018). http://papers.nips.cc/paper/7957-training-deep-models-faster-with-robust-approximate-importance-sampling.pdf
Simanshu, D. K., Nissley, D. V. & McCormick, F. RAS proteins and their regulators in human disease. Cell 170, 17–33 (2017).
Waters, A. M. & Der, C. J. KRAS: the critical driver and therapeutic target for pancreatic cancer. Cold Spring Harbor Persp. Med. 8, a031435 (2018).
Ingólfsson, H. I. et al. Machine learning-driven multiscale modeling reveals lipid-dependent dynamics of RAS signaling proteins. Preprint at Research Square https://www.researchsquare.com/article/rs-50842/v1 (2020).
Marrink, S. J., Risselada, H. J., Yefimov, S., Tieleman, D. P. & de Vries, A. H. The MARTINI Force Field: coarse grained model for biomolecular simulations. J. Phys. Chem. B 111, 7812–7824 (2007).
Di Natale, F. et al. A massively parallel infrastructure for adaptive multiscale simulations: modeling RAS initiation pathway for cancer. In Supercomputing ’19: Int. Conf. High Performance Computing, Networking, Storage, and Analysis 57 (ACM, 2019).
November 2019 TOP500 The List https://www.top500.org/lists/2019/11/ (2019).
Doersch, C. Tutorial on variational autoencoders. Preprint at https://arxiv.org/abs/1606.05908 (2016).
Jégou, H., Douze, M., Johnson, J. & Hosseini, L. FAISS. GitHub https://github.com/facebookresearch/faiss (2021).
Johnson, J., Douze, M. & Jégou, H. Billion-scale similarity search with gpus. IEEE Trans. Big Data https://doi.org/10.1109/TBDATA.2019.2921572 (2019).
Zhang, X. et al. ddcMD: a fully GPU-accelerated molecular dynamics program for the Martini Force Field. J. Chem. Phys. 153, 045103 (2020).
Correa, C., Lindstrom, P. & Bremer, P. T. Topological spines: a structure-preserving visual representation of scalar fields. IEEE Trans. Visualiz. Comput. Graph. 17, 1842–1851 (2011).
Liu, S. et al. Scalable topological data analysis and visualization for evaluating data-driven models in scientific applications. IEEE Trans. Visualiz. Comput. Graph. 26, 291–300 (2020).
Korn, F. & Muthukrishnan, S. Influence sets based on reverse nearest neighbor queries. SIGMOD Rec. 29, 201–212 (2000).
Kullback, S. & Leibler, R. A. On information and sufficiency. Ann. Math. Stat. 22, 79–86 (1951).
Marconi, U. M. B. & Tarazona, P. Dynamic density functional theory of fluids. J. Chem. Phys. 110, 8032–8044 (1999).
Tonks, M. R., Gaston, D., Millett, P. C., Andrs, D. & Talbot, P. An object-oriented finite element framework for multiphysics phase field simulations. Comput. Mater. Sci. 51, 20–29 (2012).
Streitz, F. H., Glosli, J. N. & Patel, M. V. Beyond finite-size scaling in solidification simulations. Phys. Rev. Lett. 96, 225701 (2006).
Wassenaar, T. A., Ingólfsson, H. I., Böckmann, R. A., Tieleman, D. P. & Marrink, S. J. Computational lipidomics with insane: a versatile tool for generating custom membranes for molecular simulations. J. Chem. Theor. Comput. 11, 2144–2155 (2015).
Abraham, M. J. et al. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1/2, 19–25 (2015).
Streitz, F. H. et al. 100+ TFlop solidification simulations on BlueGene/L. In Proc. 2005 ACM/IEEE Conf. Supercomputing (SC ’05) http://www.cresco.enea.it/SC05/schedule/pdf/pap307.pdf (ACM, 2005).
Glosli, J. N. et al. Extending stability beyond CPU millennium: a micron-scale atomistic simulation of Kelvin−Helmholtz instability. In Proc. 2007 ACM/IEEE Conf. Supercomputing (SC ’07) 58:1–58:11, https://doi.org/10.1145/1362622.1362700 (ACM, 2007).
Ingólfsson, H. I. et al. Capturing biologically complex tissue-specific membranes at different levels of compositional complexity. J. Phys. Chem. B 124, 7819–7829 (2020).
Acknowledgements
This work has been supported in part by the Joint Design of Advanced Computing Solutions for Cancer (JDACS4C) programme established by the US Department of Energy (DOE) and the National Cancer Institute (NCI) of the National Institutes of Health (NIH). For computing time, we thank Livermore Computing (LC) and Livermore Institutional Grand Challenge. This work was performed under the auspices of the US DOE by Lawrence Livermore National Laboratory under contract DE-AC52-07NA27344 and Los Alamos National Laboratory under contract DEAC5206NA25396. Release number: LLNL-JRNL-806073.
Author information
Authors and Affiliations
Contributions
The framework was designed by H.B., T.S.C., H.I.I., G.D., B.V.E., J.N.G., P.-T.B. and F.C.L.; the framework was implemented by H.B. Sampling analysis was done by H.B., P.K., S.L., T.O. and C.N. All authors contributed to the writing of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Machine Intelligence thanks Shangying Wang, and the other anonymous reviewer(s), for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Fig. 1 and one algorithm.
Rights and permissions
About this article
Cite this article
Bhatia, H., Carpenter, T.S., Ingólfsson, H.I. et al. Machine-learning-based dynamic-importance sampling for adaptive multiscale simulations. Nat Mach Intell 3, 401–409 (2021). https://doi.org/10.1038/s42256-021-00327-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42256-021-00327-w
This article is cited by
-
A State-of-the-Art Review on Machine Learning-Based Multiscale Modeling, Simulation, Homogenization and Design of Materials
Archives of Computational Methods in Engineering (2023)
-
Multiscale simulations of complex systems by learning their effective dynamics
Nature Machine Intelligence (2022)
-
Linking the length scales
Nature Machine Intelligence (2021)