Abstract
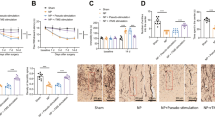
There is now a relatively large body of evidence suggesting a relationship between dysfunction of myelin and oligodendrocytes and the etiology of several neuropsychiatric disorders, including depression and schizophrenia, and also suggesting that ultrasound methods may alleviate some of the symptoms of depression. We have applied low-intensity pulsed ultrasound (LIPUS) to the brains of mice treated with the demyelinating drug cuprizone, a drug that has been used as the basis for a rodent model relevant to a number of psychiatric and neurologic disorders including depression, schizophrenia, and multiple sclerosis. Prior to conducting the studies in mice, preliminary studies were carried out on the effects of LIPUS in vitro in neuron-like SH-SY5Y cells and primary glial cells. In subsequent studies in mice, female C57BL/6 mice were restrained in plastic tubes for 20 min daily with the ultrasound transducer near the end of the tube directly above the mouse’s head. LIPUS was used at an intensity of 25 mW/cm2 once daily for 22 days in control mice and in mice undergoing daily repetitive restraint stress (RRS). Behavioral or neurochemical studies were done on the mice or the brain tissue obtained from them. The studies in vitro indicated that LIPUS stimulation at an intensity of 15 mW/cm2 delivered for 5 min daily for 3 days in an enclosed sterile cell culture plate in an incubator increased the viability of SH-SY5Y and primary glial cells. In the studies in mice, LIPUS elevated levels of doublecortin, a marker for neurogenesis, in the cortex compared to levels in the RRS mice and caused a trend in elevation of brain levels of brain-derived neurotrophic factor in the hippocampus relative to control levels. LIPUS also increased sucrose preference (a measure of the attenuation of anhedonia, a common symptom of several psychiatric disorders) in the RRS model in mice. The ability of LIPUS administered daily to rescue damaged myelin and oligodendrocytes was studied in mice treated chronically with cuprizone for 35 days. LIPUS increased cortex and corpus callosum levels of myelin basic protein, a protein marker for mature oligodendrocytes, and neural/glial antigen 2, a protein marker for oligodendrocyte precursor cells, relative to levels in the cuprizone + sham animals. These results of this exploratory study suggest that future comprehensive time-related studies with LIPUS on brain chemistry and behavior related to neuropsychiatric disorders are warranted.
Graphical abstract

Exploratory Study on Neurochemical Effects of Low Intensity Pulsed Ultrasound in Brains of Mice. Upper part of figure: LIPUS device and in-vitro cell experimental set-up. The center image is the LIPUS generating box; the image in the upper left shows the cell experiment set-up; the image in the upper right shows a zoomed-in sketch for the cell experiment; the image in the lower left shows the set-up of repetitive restraint stress (RRS) with a mouse; the image in the lower middle shows the set-up of LIPUS treatment of a mouse; the image in the lower right shows a zoomed-in sketch for the LIPUS treatment of a mouse.






Similar content being viewed by others
References
Shankar H, Pagel PS (2011) Potential adverse ultrasound-related biological effects: a critical review. Anesthesiology 115(5):1109–1124
Tyler WJ, Tufail Y, Finsterwald M, Tauchmann ML, Olson EJ, Majestic C (2008) Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound. PLoS One 3(10):e3511
Gibson BC, Sanguinetti JL, Badran BW, Yu AB, Klein EP, Abbott CC, Hansberger JT, Clark VP (2018) Increased excitability induced in the primary motor cortex by transcranial ultrasound stimulation. Front Neurol 9:1007
Wang P, Zhang J, Yu J, Smith C, Feng W (2019) Brain modulatory effects by low-intensity transcranial ultrasound stimulation (TUS): a systematic review on both animal and human studies. Front Neurosci 13:696
Tufail Y, Matyushov A, Baldwin N, Tauchmann ML, Yoshihiro GJ et al (2010) Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron 66(5):681–694
Scarcelli T, Jordao JF, O’Reilly MA, Ellens N, Hynynen K, Aubert I (2014) Stimulation of hippocampal neurogenesis by transcranial focused ultrasound and microbubbles in adult mice. Brain Stimul 7(2):304–307
Hameroff S, Trakas M, Duffield C, Annabi E, Gerace MB, Boyle P, Lucas A, Amos Q, Buadu A, Badal JJ (2013) Transcranial ultrasound (TUS) effects on mental states: a pilot study. Brain Stimul 6(3):409–415
Zhang D, Li H, Sun J, Hu W, Jin W, Li S, Tong S (2019) Antidepressant-like effect of low-intensity transcranial ultrasound stimulation. IEEE Trans Biomed Eng 66:411–420
McKinnon MC, Yucel K, Nazarov A, MacQueen GM (2009) A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci 34(1):41–54
Otsuki K, Uchida S, Watanuki T, Wakabayashi Y, Fujimoto M, Matsubara T, Funato H, Watanabe Y (2008) Altered expression of neurotrophic factors in patients with major depression. J Psychiatr Res 42(14):1145–1153
Hayley S, Poulter MO, Merali Z, Ainisman H (2005) The pathogenesis of clinical depression: stressor- and cytokine-induced alterations of neuroplasticity. Neuroscience 135(3):659–678
Edgar N, Sibille E (2012) A putative functional role for oligodendrocytes in mood regulation. Transl Psychiatry 2:e109
Wang J, Qiao J, Zhang Y, Wang H, Zhu S, Zhang H, Hartle K, Guo H, Guo W, He J, Kong J, Huang Q, Li XM (2014) Desvenlafaxine prevents white matter injury and improves the decreased phosphorylation of the rate-limiting enzyme of cholesterol synthesis in a chronic mouse model of depression. J Neurochem 131(2):229–238
Birey F, Kloc M, Chavali M, Hussein I, Wilson M, Christoffel DJ, Chen T, Frohman MA, Robinson JK, Russo SJ, Maffei A, Aguirre A (2015) Genetic and stress-induced loss of NG2 glia triggers emergence of depressive-like behaviors through reduced secretion of FGF2. Neuron 88(5):941–956
Miguel-Hidalgo JJ, Moulana M, Deloach PH, Rajkowska G (2018) Chronic unpredictable stress reduces immunostaining for connexins 43 and 30 and myelin basic protein in the rat prelimbic and orbitofrontal cortices. Chronic Stress 2:1–12
Ma T, Li B, Le Y, Xu Y, Wang F, Tian Y et al (2019) Demyelination contributes to depression comorbidity in a rat model of chronic epilepsy via dysregulation of Olig2/LINGO-1 and disturbance of calcium homeostasis. Exp Neurol 321:113034
Tang J, Liang X, Zhang Y, Chen L, Wang F, Tan C, Luo Y, Xiao Q, Chao F, Zhang L, Gao Y, Huang C, Qi Y, Tang Y (2019) The effects of running exercise on oligodendrocytes in the hippocampus of rats with depression induced by chronic unpredictable stress. Brain Res Bull 149:1–10
Vostrikov VM, Uranova NA (2020) Reduced density of oligodendrocytes and oligodendrocyte clusters in the caudate nucleus in major psychiatric illnesses. Schizophr Res 215:211–216
Zhang R, Jiang X, Chang M, Wei S, Tang Y, Wang F (2019) White matter abnormalities of corpus callosum in patients with bipolar disorder and suicidal ideation. Ann General Psychiatry 18:20
Zhang Y, Bi X, Adebiyi O, Wang J, Mooshekhian A, Cohen J, Wei Z, Wang F, Li XM (2019) Venlafaxine improves the cognitive impairment and depression-like behaviors in a cuprizone mouse model by alleviating demyelination and neuroinflammation in the brain. Front Pharmacol 10:332
Zhang Y, Xu H, Jiang W, Xiao L, Yan B, He J, Wang Y, Bi X, Li X, Kong J, Li XM (2008) Quetiapine alleviates the cuprizone-induced white matter pathology in the brain of C57BL/6 mouse. Schizophr Res 106(2-3):182–191
Sommerlad A, Price G, Trip A (2014) Management of neuropsychiatric symptoms in multiple sclerosis. Progr Neurol Psychiatry 18(2):14–19
Magavi SS, Leavitt BR, Macklis JD (2000) Induction of neurogenesis in the neocortex of adult mice. Nature 405(6789):951–955
Matrisciano F, Bonaccorso S, Ricciardi A, Scaccianoce S, Panaccione I, Wang L, Ruberto A, Tatarelli R, Nicoletti F, Girardi P, Shelton RC (2009) Changes in BDNF serum levels in patients with major depression disorder (MDD) after 6 months treatment with sertraline, escitalopram, or venlafaxine. J Psychiatr Res 43(3):247–254
Molteni R, Calabrese F, Cattaneo A, Mancini M, Gennarelli M, Racagni G, Riva MA (2009) Acute stress responsiveness of the neurotrophin BDNF in the rat hippocampus is modulated by chronic treatment with the antidepressant duloxetine. Neuropsychopharmacology 34(6):1523–1532
Doron R, Lotan D, Versano Z, Benatav L, Franko M, Armoza S et al (2014) Escitalopram or novel herbal mixture treatments during or following exposure to stress reduce anxiety-like behavior through corticosterone and BDNF modifications. PLoS One 9(4):e91455
Engel D, Zomkowski AD, Lieberknecht V, Rodrigues AL, Gabilan NH (2013) Chronic administration of duloxetine and mirtazapine downregulates proapoptotic proteins and upregulates neurotrophin gene expression in the hippocampus and cerebral cortex of mice. J Psychiatr Res 47(6):802–808
Freire TFV, Fleck MP, da Rocha NS (2016) Remission of depression following electroconvulsive therapy (ECT) is associated with higher levels of brain-derived neurotrophic factor (BDNF). Brain Res Bull 121:263–269
Gedge L, Beaudoin A, Lazowski L, du Toit R, Jokic R, Milev R (2012) Effects of electroconvulsive therapy and repetitive transcranial magnetic stimulation on serum brain-derived neurotrophic factor levels in patients with depression. Front Psychiatry 3:12
Husain M, Roiser JP (2018) Neuroscience of apathy and anhedonia: a transdiagnostic approach. Nat Rev Neuurosci 19:470–484
Romer Thomsen K, Whybrow PC, Kringelbach ML (2015) Reconceptualizing anhedonia: novel perspectives on balancing the pleasure networks in the human brain. Front Behav Neurosci 9:49
Wang H, Xu H, Niu J, Mei F, Li X, Kong J, Cai W, Xiao L (2010) Haloperidol activates quiescent oligodendroglia precursor cells in the adult mouse brain. Schizophr Res 119(1-3):164–174
Qiao J, Wang J, Wang H, Zhang Y, Zhu S, Adilijiang A et al (2016) Regulation of astrocyle pathology by fluoxetine prevents the deterioration of Alzheimer phenotypes in an APP/PS1 mouse model. Glia 64(2):240–254
Zhu S, Shi R, Wang J, Wang JF, Li XM (2014) Unpredictable chronic mild stress not chronic restraint stress induces depressive behaviours in mice. Neuroreport 25(14):1151–1155
Pascual-Leone A, Dolores Catala M, Pascual-Leone Pascual A (1996) Lateralized effect of rapid-rate transcranial magnetic stimulation of the prefrontal cortex on mood. Neurology 46(2):499–502
George MS, Johnson RH, Taylor JJ, Short EB (2013) The expanding evidence base for rTMS treatment of depression. Curr Opin Psychiatry 26(1):13–18
Berlim MT, den Dynde V, Daskalakis ZJ (2013) Clinically meaningful efficacy and acceptability of low-frequency repetitive transcranial magnetic stimulation (rTMS) for treating primary major depression: a meta-analysis of randomized, double-blind and sham-controlled trials. Neuropsychopharmacology 38:543–551
Janicak PG, Dokucu ME (2015) Transcranial magnetic stimulation for the treatment of major depression. Neuropsychiatr Dis Treat 11:1549–1560
Kar SK (2019) Predictors of response to repetitive transcranial magnetic stimulation in depression: a review of recent updates. Clin Psychopharmacol Neurosci 17(1):25–33
Kedzior KK, Reitz SK (2014) Short-term efficacy of repetitive transcranial magnetic stimulation (rTMS) in depression-reanalysis of data from meta-analyses up to 2010. BMC Psychol 2(1):39
McClintock SM, Reti IM, Carpenter LL, McDonald WM, Dubin M, Taylor SF et al (2018) Concensus recommendations for the clinical application of repetitive transcranial magentic stimulation (rTMS) in the treatment of depression. J Clin Psychiatry 79(1):16cs10905
Bachtold MR et al (1998) Focused ultrasound modifications of neural circuit activity in a mammalian brain. Ultrasound Med Biol 24(4):557–565
King RL, Brown JR, Newsome WT, Pauly KB (2013) Effective parameters for ultrasound-induced in vivo neurostimulation. Ultrasound Med Biol 39(2):312–331
Legon W, Sato TF, Opitz A, Mueller J, Barbour A, Williams A, Tyler WJ (2014) Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nat Neurosci 17(2):322–329
Xu P, Gul-Uludag H, Ang WT, Yang X, Huang M, Marquez-Curtis L, McGann L, Janowska-Wieczorek A, Xing J, Swanson E, Chen J (2012) Low-intensity pulsed ultrasound-mediated stimulation of hematopoietic stem/progenitor cell viability, proliferation and differentiation. Biotechnol Lett 34(10):1965–1973
Zhao Y, Xing J, Xing JZ, Ang WT, Chen J (2014) Applications of low-intensity pulse ultrasound to increase monoclonal antibody production in CHO cells using shake flasks or wavebags. Ultrasonics 54(6):1439–1447
Leung KS, Lee WS, Tsui HF, Liu PP, Cheung WH (2004) Complex tibial fracture outcomes following treatment with low-intensity pulsed ultrasound. Ultrasound Med Biol 30(3):389–395
Ohira K, Takeuchi R, Shoji H, Miyakawa T (2013) Fluoxetine-induced cortical adult neurogenesis. Neuropsychopharmacology 38:909–920
Jaako-Movits K, Zharkovsky T, Pedersen M, Zharkovsky A (2006) Decreased hippocampal neurogenesis following olfactory bulbectomy is reversed by repeated citalopram administration. Cell Mol Neurobiol 26(7/8):1559–1570
Madsen TM, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingstrom A (2000) Increased neurogenesis in a model of electroconvulsive therapy. Biol Psychiatry 47(12):1043–1049
Ueyama E, Ukai S, Ogawa A, Yamamoto M, Kawaguchi S, Ishii R, Shinosaki K (2011) Chronic repetitive transcranial magnetic stimulation increases hippocampal neurogenesis in rats. Psychiatry Clin Neurosci 65(1):77–81
Czeh B, Michaelis T, Watanabe T, Frahm J, de Birrun G, van Kampen M et al (2001) Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci U S A 98(22):12796–12801
Petrik D, Lagace DC, Eisch AJ (2012) The neurogenesis hypothesis of affective and anxiety disorders: are we mistaking the scaffolding for the building? Neuropharmacology 62(1):21–34
Peng Q, Masuda N, Jiang M, Li Q, Zhao M, Ross CA, Duan W (2008) The antidepressant sertraline improves the phenotype, promotes neurogenesis and increases BDNF levels in the R6/2 Huntington’s disease mouse model. Exp Neurol 210(1):154–163
Mateus-Pinheiro A, Patricio P, Alves ND, Machado-Santos AR, Morais M, Bessa JM et al (2014) The Sweet Drive Test: refining phenotypic characterization of anhedonic behavior rodents. Front Behav Neurosci 8:74
Hageman I, Nielsen M, Wortwein G, Diemer NH, Jorgensen MB (2009) Electroconvulsive stimulations normalizes stress-induced changes in the glucocorticoid receptor and behaviour. Behav Brain Res 196(1):71–77
Ampuero E, Luarte A, Santibanez M, Varas-Godoy M, Toledo J, Diaz-Veliz G et al (2015) Two chronic stress models based on movement restriction in rats respond selectively to antidepressant drugs: aldolase C as a potential biomarker. Int J Neuropsychopharmacol 18(10):pyv038
Kuroda Y, McEwen BS (1998) Effect of chronic restraint stress and tianeptine on growth factors, growth-associated protein-43 and microtubule-associated protein 2 mRNA expression in the rat hippocampus. Brain Res Mol Brain Res 59(1):35–39
Yamaura K, Bi Y, Ishiwatari M, Oishi N, Fukata H, Ueno K (2013) Sex differences in stress reactivity of hippocampal BDNF in mice are associated with the female preponderance of decreased locomotor activity in response to restraint stress. Zool Sci 30(12):1019–1024
Maghsoudi N, Ghasemi R, Ghaempanah Z, Ardekani M, Nooshinfar E, Tahzibi A (2014) Effect of chronic restraint stress on HPA axis activity and expression of BDNF and Trkb in the hippocampus of pregnant rats: possible contribution in depression during pregnancy and postpartum period. Basic Clin Neurosci 5(2):131–137
Willner P (2005) Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 52(2):90–110
Murphy R, O’Donoghue S, Counihan T, McDonald C, Calabresi PA, Ahmed MAS (2017) Neuropsychiatric syndromes of multiple sclerosis. J Neurol Neurosurg Psychiatry 88(8):697–708
Hemanth Kumar BS, Mishra SK, Trivedi R, Singh S, Rana P, Khushu S (2014) Demyelinating evidences in CMS rat model of depression: a DTI study at 7T. Neuroscience 275:12–21
Nawaz S, Schweitzer J, Jahn O, Werner HB (2013) Molecular evolution of myelin basic protein, an abundant structural myelin component. Glia 61:1364–1377
Xiao L, Xu H, Zhang Y, Wei Z, He J, Jian W et al (2008) Quetiapine facilitates oligodendrocyte development and prevents mice from myelin breakdown and behavioral changes. Mol Psychiatry 13(7):697–708
Zhang Y, Zhang H, Wang L, Jiang W, Xu H, Xiao L, Bi X, Wang J, Zhu S, Zhang R, He J, Tan Q, Zhang D, Kong J, Li XM (2012) Quetiapine enhances oligodendrocyte regeneration and myelin repair after cuprizone-induced demyelination. Schizophr Res 138(1):8–17
Forbes TA, Gallo V (2017) All wrapped up: environmental effects on myelination. Trends Neurosci 40(9):572–587
Kuhn S, Gritti L, Crooks D, Dombrowski Y (2019) Oligodendrocytes in development, myelin generation and beyond. Cells 8:1424
Tsiperson S, Huang Y, Bagayogo I, Song Y, VonDran MW, DiCicco-Bloom E, Dreyfus CF (2015) Brain-derived neurotrophic factor deficiency restricts proliferation of oligodendrocyte progenitors following cuprizone-induced demyelination. ASN Neuro 7(1):1–11
Tripathi RB, Rivers LE, Young KM, Jamen F, Richardson WD (2010) NG2 glia generate new oligodendrocytes but few astrocytes in a murine experimental autoimmune encephalomyelitis model of demyelinating diseases. J Neurosci 30(48):16383–16390
Kipp M, Clarner T, Dang J, Copray S, Beyer C (2009) The cuprizone animal model: new insights into an old story. Acta Neuropathol 118:723–736
Skripuletz T, Gudi V, Haackstette D, Stangel M (2011) De- and remyelination in the CNS white and grey matter induced by cuprizone: the old, the new, and the unexpected. Histol Histopathol 26:1585–1597
Theissen JD, Zhang Y, Zhang H, Wang L, Buist R, Del Bigio MR, Kong J, Li X-M, Martin M (2013) Quantitative MRI and ultrastructural examination of the cuprizone mouse model of demyelination. NMR Biomed 26:1562–1581
Elsayed M, Banasr M, Duric V, Fournier NM, Licznerski P, Duman RS (2012) Antidepressant effects of fibroblast growth factor-2 in behavioral and cellular models of depression. Biol Psychiatry 72(4):258–265
Wennstrom M, Hellsten J, Ekdahl CT, Tingstrom A (2003) Electroconvulsive seizures induce proliferation of NG2-expressing glial cells in adult rat hippocampus. Biol Psychiatry 54(10):1015–1024
Wennstrom M, Hellsten J, Tingstrom A (2004) Electroconvulsive seizures induce proliferation of NG2-expressing glial cells in adult rat amygdala. Biol Psychiatry 55(5):464–471
Petković F, Campbell IL, Gonzalez B, Castellano B (2016) Astrocyte-targeted production of interleukin-6 reduces astroglial and microglial activation in the cuprizone demyelination model: implications for myelin clearance and oligodendrocyte maturation. Glia 64(12):2104–2119
Yoon H, Choi CI, Triplet EM, Langley MR, Kleppe LS, Kim HN, Simon WL, Scarisbrick IA (2020) Blocking the thrombin receptor promotes repair of demyelinated lesions in the adult brain. J Neurosci 40(7):1483–1500
Kalakh S, Mouihate A (2017) Androstenediol reduces demyelination- induced axonopathy in the rat corpus callosum: impact on microglial polarization. Front Cell Neurosci 11:49
Funding
Natural Sciences and Engineering Research Council of Canada (NSERC) (JC) and the Capital Health Chair in Mental Health (XL). The figures in this paper were taken from the MSc thesis of Huining Guo (University of Alberta), with her permission; Huining is the first author on this paper.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Clinical impact statement: There is an urgent need for more effective therapy of a number of neuropsychiatric disorders, including depression and schizophrenia, and the exploratory neurochemical and behavioral findings in the study reported here suggest that LIPUS may be a useful, relatively non-invasive adjunct treatment for these serious mental illnesses by affecting factors involved in neurogenesis and myelination.
Rights and permissions
About this article
Cite this article
Guo, H., Baker, G., Hartle, K. et al. Exploratory study on neurochemical effects of low-intensity pulsed ultrasound in brains of mice. Med Biol Eng Comput 59, 1099–1110 (2021). https://doi.org/10.1007/s11517-021-02351-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-021-02351-9