Abstract
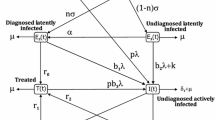
Tuberculosis has continued to retain its title as “the captain among these men of death”. This is evident as it is the leading cause of death globally from a single infectious agent. TB as it is fondly called has become a major threat to the achievement of the sustainable development goals (SDG) and hence require inputs from different research disciplines. This work presents a mathematical model of tuberculosis. A compartmental model of seven classes was used in the model formulation comprising of the susceptible S, vaccinated V, exposed E, undiagnosed infectious I1, diagnosed infectious I2, treated T and recovered R. The stability analysis of the model was established as well as the condition for the model to undergo backward bifurcation. With the existence of backward bifurcation, keeping the basic reproduction number less than unity \(({R_{0}}<1)\) is no more sufficient to keep TB out of the community. Hence, it is shown by the analysis that vaccination program, diagnosis and treatment helps to control the TB dynamics. In furtherance to that, it is shown that preference should be given to diagnosis over treatment as diagnosis precedes treatment. It is as well shown that at lower vaccination rate (0–20%), TB would still be endemic in the population. As such, high vaccination rate is required to send TB out of the community.




















Similar content being viewed by others
References
Agusto FB, Khan MA (2018) Optimal control strategies for dengue transmission in Pakistan. Math Biosci 305:102–121
Ayinla AY, Wan Ainun MO (2019) A compartmental model on the effect of quarantine on MDR-TB. Int J Math Comput Sci 14:613–629
Ayinla AY, Wan Ainun MO, Omar Awang MA (2019) The role of vaccination in curbing tuberculosis epidemic. Model Earth Syst Environ 5:1689–1704
Benjamin HS, Denise EK (2004) Influence of backward bifurcation on interpretation of \(R_{0}\) in a model of epidemic tuberculosis with reinfection. Math Biosci Eng 1:81–93
Bhunu C, Garira W, Mukandavire Z, Zimba M (2008) Tuberculosis transmission model with chemoprophylaxis and treatment. Bull Math Biol 70:1163–1191
Bhunu CP, Mushayabasa S, Tchuenche JM (2011) A theoretical assessment of the effects of smoking on the transmission dynamics of tuberculosis. Bull Math Biol 73:1333–1357
Blower SM, Mclean AR, Porco TC, Small PM, Hopewell PC, Sanchez MA, Moss AR (1995) The intrinsic transmission dynamics of tuberculosis epidemics. Nat Med 1:815–821
Castillo-Chavez C, Feng Z (1997) To treat or not to treat: the case of tuberculosis. J Math Biol 35:629–656
Castillo-Chavez C, Song B (2004) Dynamical models of tuberculosis and their applications. Math Biosci Eng 1:361–404
Castillo-Chavez C, Feng Z, Huang W (2002) On the computation of \(R_{0}\) and its role on global stability. Math Approach Emerg Reemerg Infect Dis 1:229–250
Choi S, Jung E, Lee SM (2015) Optimal intervention strategy for prevention tuberculosis using a smoking-tuberculosis model. J Theor Biol 380:256–270
Cohen T, Colijn C, Finklea B, Murray M (2007) Exogenous re-infection and the dynamics of tuberculosis epidemics: local effects in a network model of transmission. J R Soc Interface 4:523–531
Colijn C, Cohen T, Murray M (2007) Emergent heterogeneity in declining tuberculosis epidemics. J Theor Biol 247:765–774
Diekmann O, Heesterbeek JAP, Metz JA (1990) On the definition and the computation of the basic reproduction ratio \(R_{0}\) in models for infectious diseases in heterogeneous populations. J Math Biol 28:365–382
Feng Z, Castillo-Chavez C, Capurro AF (2000) A model for tuberculosis with exogenous reinfection. Theor Popul Biol 57:235–247
Flynn JN, Chan J (2001) Tuberculosis: latency and reactivation. Infect Immun 69:4195–4201
Gomes MGM, Franco AO, Gomes MC, Medley GF (2004) The reinfection threshold promotes variability in tuberculosis epidemiology and vaccine efficacy. Proc R Soc Lond B 271:617–623
Goswami NK, Srivastav AK, Ghosh M, Shanmukha B (2018) Mathematical modeling of zika virus disease with nonlinear incidence and optimal control. J Phys 1000:1–16
Gumel AB (2012) Causes of backward bifurcation in some epidemiological models. J Math Anal Appl 395:355–365
Hadeler KP, van den Driessche P (1997) Backward bifurcation in epidemic control. Math Biosci 146:15–35
Hethcote HW (2000) The mathematics of infectious diseases. SIAM Rev 42:599–653
Huo HF, Zou MX (2016) Modelling effects of treatment at home on tuberculosis transmission dynamics. Appl Math Model 40:9474–9484
Moualeu DP, Weiser M, Ehrig R, Deuflhard P (2015) Optimal control for a tuberculosis model with undetected cases in Cameroon. Commun Nonlinear Sci Num Simul 20:986–1003
Moualeu DP, Yakam AN, Bowong S, Temgoua A (2016) Analysis of a tuberculosis model with undetected and lost-sight cases. Commun Nonlinear Sci Num Simul 41:48–63
Okuonghae D, Ikhimwin BO (2016) Dynamics of a mathematical model for tuberculosis with variability in susceptibility and disease progressions due to difference in awareness level. Front Microbiol 6:1–23
Okuonghae D, Korobeinikov A (2007) Dynamics of tuberculosis: the effect of direct observation therapy strategy (DOTS) in Nigeria. Math Model Nat Phenomena 2:113–128
Okuonghae D, Omosigho SE (2011) Analysis of a mathematical model for tuberculosis: what could be done to increase case detection. J Theor Biol 269:31–45
Sharomi O, Gumel AB (2009) Re-infection-induced backward bifurcation in the transmission dynamics of Chlamydia trachomatis. J Math Anal Appl 356:96–118
Sharomi O, Podder C, Gumel A, Song B (2008) Mathematical analysis of the transmission dynamics of HIV/TB coinfection in the presence of treatment. Math Biosci Eng 5:145–174
Srivastav AK, Ghosh M (2016) Modeling and analysis of the symptomatic and asymptomatic infections of swine flu with optimal control. Model Earth Syst Environ 2:1–9
Ullah S, Khan MA, Farooq M, Gul T (2019) Modeling and analysis of tuberculosis (TB) in Khyber Pakhtunkhwa, Pakistan. Math Comput Simul 165:181–199
United Nations (2019) The sustainable development goals Report. United Nations, New York
van den Driessche P, Watmough J (2002) Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math Biosci 180:29–48
Wangari IM, Stone L (2018) Backward bifurcation and hysteresis in models of recurrent tuberculosis. PLoS ONE 13:1–29
WHO (2018) Global tuberculosis report. WHO Report, Geneva
WHO (2018) WHO preferred product characteristics for new tuberculosis vaccines. WHO, Geneva
WHO (2019) Global tuberculosis report. WHO Report, Geneva
Xiang H, Zou MX, Huo HF (2019) Modeling the effects of health education and early therapy on tuberculosis transmission dynamics. Int J Nonlinear Sci Num Simul 20:243–255
Yang HM, Raimundo SM (2010) Assessing the effects of multiple infections and long latency in the dynamics of tuberculosis. Theor Biol Med Model 7:1–37
Zhang X, Liu X (2008) Backward bifurcation of an epidemic model with saturated treatment function. J Math Anal Appl 348:433–443
Zhang X, Liu X (2009) Backward bifurcation and global dynamics of an SIS epidemic model with general incidence rate and treatment. Nonlinear Anal 10:565–575
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ayinla, A.Y., Othman, W.A.M. & Rabiu, M. A Mathematical Model of the Tuberculosis Epidemic. Acta Biotheor 69, 225–255 (2021). https://doi.org/10.1007/s10441-020-09406-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10441-020-09406-8