Abstract
Feasibility of dechlorination using ultra-high lime with aluminum process (UHLA) has been demonstrated by previous studies. However, the negative influences of UHLA on water qualities (pH, conductivity, and residual calcium concentration) after dechlorination and the products generated in dechlorination process have not been thoroughly analyzed. In this study, changes of water qualities at various dechlorination conditions were investigated, and favorable conditions for Friedel’s salt formation were explored by X-ray diffraction analysis. The pH and conductivity after dechlorination increased with the improvement of chloride removal effect. The calcium ion concentration in effluent also fluctuated with different dosages of CaO. At the maximum chloride removal efficiency of 71.4%, the pH, conductivity, and calcium ion concentration in effluent were 12.8, 10.9 ms/cm, and 22.7 mg/L, respectively. X-ray diffraction analysis for precipitants indicated that Friedel’s salt with the highest purity was also generated at the optimal dechlorination efficiency. The high dechlorination efficiency of 80.5% was achieved with the development of a stepwise addition strategy. Chemical costs for treating one ton of wastewater containing 500 mg/L chloride were $3.96.
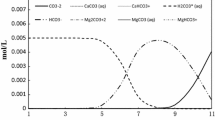
Graphical abstract












Similar content being viewed by others
References
Abdel-Wahab, A., & Batchelor, B. (2006). Effects of pH, temperature, and water quality on chloride removal with ultra-high lime with aluminum process. Water Environment Research, 78(9), 930–937.
Abdel-Wahab, A., & Batchelor, B. (2013). Evaluating alternative aluminium sources for chloride removal from recycled cooling water. International Journal of Environmental Technology and Management, 16(3), 234–243.
Abdel-Wahab, A., Batchelor, B., & Schwantes, J. (2005). An equilibrium model for chloride removal from recycled cooling water using the ultra-high lime with aluminum process. Water Environment Research, 77(7), 3059–3065.
Artiga, P., Garcia-Toriello, G., Mendez, R., & Garrido, J. M. (2008). Use of a hybrid membrane bioreactor for the treatment of saline wastewater from a fish canning factory. Desalination, 221(1–3), 518–525.
Chang, J., Li, Y., Duan, F., Su, C., Li, Y., & Cao, H. (2020). Selective removal of chloride ions by bismuth electrode in capacitive deionization. Separation and Purification Technology, 240(116600), 1–8.
Darwish, M. A., Abdel-Jawad, M., & Hauge, L. J. (1989). A new dual-function device for optimal energy recovery and pumping for all capacities of RO systems. Desalination, 75, 25–39. https://doi.org/10.1016/0011-9164(89)85003-9.
Ellwood, M. J., Hunter, K. A., & Cunninghame, R. G. (2003). Elimination of equivalence point errors in the potentiometric titration of chloride, bromide and iodide mixtures with silver nitrate. Microchemical Journal, 74(2), 187–192.
Goni, S., & Guerrero, A. (2003). Accelerated carbonation of Friedel’s salt in calcium aluminate cement paste. Cement and Concrete Research, 33(1), 21–26.
Hamoda, M. F., & Al-Attar, I. M. S. (1995). Effects of high sodium chloride concentrations on activated sludge treatment. Water Science and Technology, 31(9), 61–72.
Ito, R., Dodbiba, G., Fujita, T., & Ahn, J. W. (2008). Removal of insoluble chloride from bottom ash for recycling. Waste Management, 28(8), 1317–1323.
Kameda, T., Yabuuchi, F., Yoshioka, T., Uchida, M., & Okuwaki, A. (2003). New method of treating dilute mineral acids using magnesium-aluminum oxide. Water Research, 37(7), 1545–1550.
Kukli, K., Mikkor, M., Sutka, A., Kull, M., Seemen, H., Link, J., et al. (2020). Behavior of nanocomposite consisting of manganese ferrite particles and atomic layer deposited bismuth oxide chloride film. Journal of Magnetism and Magnetic Materials, 498(166167), 1–6.
Lefebvre, O., Vasudevan, N., Torrijos, M., Thanasekaran, K., & Moletta, R. (2006). Anaerobic digestion of tannery soak liquor with an aerobic post-treatment. Water Research, 40(7), 1492–1500.
Lv, L. A., He, J., Wei, M., Evans, D. G., & Duan, X. (2006). Uptake of chloride ion from aqueous solution by calcined layered double hydroxides: Equilibrium and kinetic studies. Water Research, 40(4), 735–743.
Ma, J., Li, Z., Zhang, Y., & Demopoulos, G. P. (2009). Desilication of sodium aluminate solution by Friedel’s salt (FS: 3CaO•A12O3•CaCl2•10H2O). Hydrometallurgy, 99(3), 225–230. https://doi.org/10.1016/j.hydromet.2009.08.010.
Novotny, E. V., & Stefan, H. G. (2010). Projections of chloride concentrations in urban lakes receiving road de-icing salt. Water Air and Soil Pollution, 211(1–4), 261–271.
Panswad, T., & Anan, C. (1999). Impact of high chloride wastewater on an anaerobic/anoxic/aerobic process with and without inoculation of chloride acclimated seeds. Water Research, 33(5), 1165–1172.
Park, Y.-M., Yeon, K.-M., & Park, C.-h. (2020). Silica treatment technologies in reverse osmosis for industrial desalination: A review. Environmental Engineering Research, 25(6), 819–829.
Shen, Y., Tong, Y., Xu, J., Wang, S., Wang, J., Zeng, T., et al. (2020). Ni-based layered metal-organic frameworks with palladium for electrochemical dechlorination. Applied Catalysis B-Environmental, 264(118505), 1–10.
Song, R. P., Hou, Y. X., Zhang, Y. P., Zhou, Y. C., & Zhang, T. Q. (2020). Salinity distribution and sediment flux in the estuarine Xuanmen reservoir. Water Air and Soil Pollution, 231(6), 1–10.
Suryavanshi, A. K., Scantlebury, J. D., & Lyon, S. B. (1996). Mechanism of Friedel’s salt formation in cements rich in tri-calcium aluminate. Cement and Concrete Research, 26(5), 717–727. https://doi.org/10.1016/S0008-8846(96)85009-5.
Vitezova, M., Vitez, T., & Nitayapat, N. (2016). Effects of de-icing salts on the respiration of the microorganisms of activated sludge. Water Air and Soil Pollution, 227(11), 1–7.
Wang, L., Lee, W.-H., Tseng, S., & Cheng, T.-W. (2017). Removal of chloride ions from an aqueous solution containing a high chloride concentration through the chemical precipitation of Friedel’s salt. Materials Transactions, 59(2), 297–302.
Yao, J., Kong, Q., Zhu, H., Long, Y., & Shen, D. (2014). Adsorption characteristics of nitrite on Friedel’s salt under the landfill circumstance. Chemical Engineering Journal, 254, 479–485.
Zhang, D., Jia, Y., Ma, J., & Li, Z. (2011). Removal of arsenic from water by Friedel’s salt (FS: 3CaO center dot Al2O3 center dot CaCl2 center dot 10H(2)O). Journal of Hazardous Materials, 195, 398–404.
Zhu, Z., Zhang, W., & Cheng, C. Y. (2012). A synergistic solvent extraction system for separating copper from iron in high chloride concentration solutions. Hydrometallurgy, 113, 155–159.
Zou, L., Morris, G., & Qi, D. (2008). Using activated carbon electrode in electrosorptive deionisation of brackish water. Desalination, 225(1–3), 329–340.
Funding
This work was supported by the National Key R&D Program of China, the High-efficient Development and Utilization of Water Resource (2019YFC0408202), and the National Natural Science Foundation of China (NSFC) (21876050).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. Conductivity and pH in effluent increased after UHLA dechlorination process.
2. About 22.7–352 mg/L Ca2+ was found in effluent after dechlorination.
3. More Friedel’s salt was produced at maximum chloride removal efficiency.
4. Stepwise addition strategy was developed to improve dechlorination efficiency of 80.5%.
Supplementary information
ESM 1
(DOCX 2.40 mb)
Rights and permissions
About this article
Cite this article
Xu, J., Chen, P., Dou, C. et al. Water Qualities and Products Generated in Dechlorination Process Using Ultra-high Lime with Aluminum Method. Water Air Soil Pollut 232, 170 (2021). https://doi.org/10.1007/s11270-021-04996-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-04996-6