Abstract
Backround
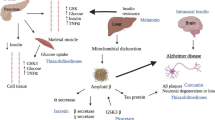
Both type 2 diabetes (T2D) and Alzheimer’s disease affect large number of people all over the world, especially in developed countries, and involve common molecular mechanisms in degenerative processes.
Aim
Our study is to investigate the expression levels of the ADAMTS4, TIMP3, RELN, and BCAN genes which encode extracellular matrix molecules and are thought to be related to the pathophysiology of Alzheimer’s disease in rats that we used to develop a T2D model by injection of a streptozotocin (STZ) and feeding with high-fat diet (HFD).
Material and methods
In total, 40 rats were divided into four groups: HFD + STZ (10), HFD (10), STZ (10), and the control (10). The weight and blood glucose levels of all rats were recorded, and the insulin tolerance test was performed. At the end of the experimental period, the hippocampus of the rats was isolated and a part of this was used for gene expression analysis through real-time PCR, whereas other parts were used for histological analyses.
Results
Our results show that plaque-like structures were found in the histological examination of the experimental T2D model. In molecular studies, the expression levels of the ADAMTS4, TIMP3, RELN, and BCAN genes were decreased in the HFD +STZ and STZ groups compared with the control, whereas the same expression levels, except that of inhibitor TIMP3, were found to increase in the HFD group.
Conclusion
These genes encoding proteases that regulate neuronal activity have decreased levels in the T2D model. There may be potential in the adoption of new treatment approaches for Alzheimer’s and T2D.




Similar content being viewed by others
References
Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81.
Ley SH, Meigs JB. Epidemiology and risk factors of type 2 diabetes. Switzerland: Endocrinology; 2018.
Introduction. Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41(1):S1–2.
Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(11):2065–79.
Goldstein BJ, Müller-Wieland D. Type 2 diabetes. 2nd ed. USA: Informa Healthcare; 2008. p. 13–26.
Kyrou I, Tsigos C. Obesity in the elderly diabetic patient: ıs weight loss beneficial? No. Diabetes Care. 2009;32(2):403–9.
Goyal R, Jialal I. Diabetes mellitus type 2. Treasure Island: StatPearls; 2020.
Pulgaron ER, Delamater AM. Obesity and type 2 diabetes in children: epidemiology and treatment. Curr Diab Rep. 2014;14(8):508.
Ridge PG, Ebbert MT, Kauwe JS. Genetics of Alzheimer’s disease. Biomed Res Int. 2013;25:254954.
Ciudin A. Diabetes mellitus and Alzheimer’s disease: an unforgettable relation. Endocrinol Nutr. 2016;93(5):191–3.
Cole AR, Astell A, Green C, Sutherland C. Molecular connexions between dementia and diabetes. Neurosci Biobehav Rev. 2007;3:1046–63.
Miyata S, Kitagawa H. Formation and remodeling of the brain extracellular matrix in neural plasticity: roles of chondroitin sulfate and hyaluronan. Biochim Biophys Acta. 2017;1861(10):2420–34.
Gottschall PE, Howell MD. ADAMTS expression and function in central nervous system injury and disorders. Matrix Biol. 2015;44-46:70–6.
Morawski M, Filippov M, Tzinia A, Tsilibary E, Vargova L. ECM in brain aging and dementia. Prog Brain Res. 2014;214:207–27.
Frischknecht R, Happel MFK. Impact of the extracellular matrix on plasticity in juvenile and adult brains. e-Neuroforum. 2016;7:1–6. https://doi.org/10.1007/s13295-015-0021-z January 13,2021.
Qinna NA, Badwan AA. Impact of streptozotocin on altering normal glucose homeostasis during insulin testing in diabetic rats compared to normoglycemic rats. Drug Des Devel Ther. 2015;9:2515–25.
Pentkowski NS, Litvin Y, Blanchard DC, Vasconcellos A, King LB, Blanchard RJ. Effects of acidic-astressin and ovine-CRF microinfusions into the ventral hippocampus on defensive behaviors in rats. Horm Behav. 2009;56(1):35–43.
Hazman Ö, Ovalı S. Investigation of the anti-ınflammatory effects of safranal on high-fat diet and multiple low-dose streptozotocin ınduced type 2 diabetes rat model. Inflammation. 2015;38(3):1012–9.
Okamoto T, Kanemoto N, Ohbuchi Y, Okano M, Fukui H, Sudo T. Characterization of STZ-ınduced type 2 diabetes in Zucker fatty rats. Exp Anim. 2008;57(4):335–45.
Sandhir R, Gupta S. Molecular and biochemical trajectories from diabetes to Alzheimer’s disease: a critical appraisal. World J Diabetes. 2015;6(12):1223–42.
De la Monte SM, Wands JR. Alzheimer’s disease is type 3 diabetes evidence reviewed. J Diabetes Sci Technol. 2008;2:1101–13.
Mittal K. Katare DP Shared links between type 2 diabetes mellitus and Alzheimer’s disease: a review. Diabetol Metab Syndr. 2016;10(2):144–9.
Qin L, Reddy PH. Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochim Biophys Acta. 2017;1863(5):1037–45.
Biessels GJ, Kappelle LJ. Increased risk of Alzheimer’s disease in type II diabetes: insulin resistance of the brain or insulin-induced amyloid pathology? Biochem Soc Trans. 2005;33(5):1041–4.
Lemarchant S, Pruvost M, Montaner J, Emery E, Vivien D, Kanninen K, et al. ADAMTS proteoglycanases in the physiological and pathological central nervous system. J Neuroinflammation. 2013;10:133.
Satoh K, Suzuki N, Yokota H. ADAMTS-4 (a disintegrin and metalloproteinase with thrombospondin motifs) is transcriptionally induced in beta-amyloid treated rat astrocytes. Neurosci Lett. 2000;289:177–80.
Gurses MS, Ural MN, Gulec MA, Akyol O, Akyol S. Pathophysiological function of ADAMTS enzymes on molecular mechanism of Alzheimer’s disease. Aging Dis. 2016;7(4):479–90.
Lemarchant S, Pomeshchik Y, Kidin I, Kärkkäinen V, Valonen P, Lehtonen S, et al. ADAMTS-4 promotes neurodegeneration in a mouse model of amyotrophic lateral sclerosis. Mol Neurodegener. 2016;11:10.
Pehlivan S, Fedakar R, Eren B, Akyol S, Eren F, Turkmen Inanır N, et al. ADAMTS4, 5, 9, and 15 expressions in the autopsied brain of patients with Alzheimers disease: a preliminary immünohistochemistry study. Bull Clin Psychopharmacol. 2016;26(1):7–14.
Gibb SL, Zhao Y, Potter D, Hylin MJ, Bruhn R, Baimukanova G, et al. TIMP3 attenuates the loss of neural stem cells, mature neurons and neurocognitive dysfunction in traumatic brain ınjury. Stem Cells. 2015;33(12):3530–44.
Yu NN, Tan MS, Yu JT, Xie AM, Tan L. The role of reelin signaling in Alzheimer’s disease. Mol Neurobiol. 2016;53(8):5692–700.
Krstic D, Rodriguez M, Knuesel I. Regulated proteolytic processing of reelin through interplay of tissue plasminogen activator (tPA), ADAMTS-4, ADAMTS-5, and their modulators. PLoS One. 2012;7(10):47793.
Saez-Valero J, Costell M, Sjogren M, Andreasen N, Blennow K. Luque JM Altered levels of cerebrospinal fluid reelin in frontotemporal dementia and Alzheimer’s disease. J Neurosci Res. 2003;72(1):132–6.
Gary SC, Zerillo CA, Chiang VL, Gaw JU, Gray G, Hockfield S. cDNA cloning, chromosomal localization, and expression analysis of human BEHAB/brevican, a brain specific proteoglycan regulated during cortical development and in glioma. Gene. 2000;256(1-2):139–47.
Valenzuela JC, Heise C, Franken G, Singh J, Schweitzer B, Seidenbecher CI, et al. Hyaluronanbased extracellular matrix under conditions of homeostatic plasticity. Philos Trans R Soc Lond Ser B Biol Sci. 2014;369(1654):20130606.
Morawski M, Brückner G, Jäger C, Seeger G, Matthews RT, Arendt T. Involvement of perineuronal and perisynaptic extracellular matrix in Alzheimer’s disease neuropathology. Brain Pathol. 2012;22(4):547–61.
Funding
This work was supported by “Cukurova University Research Projects Funding Unit” with project number 2016-5883.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Lütfiye Özpak, Ayfer Pazarbasi, Işıl Öcal, M. Bertan Yılmaz, and Hülya Binokay. The first draft of the manuscript was written by Lütfiye Özpak and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Throughout the study, all interventions applied to the animals were carried out in accordance with the approval (reference number: ÇÜTF-DETAUM-09; date: 27.11.2015) of Çukurova University Animal Experiments Local Ethics Committee.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Özpak, L., Pazarbasi, A., Öcal, I. et al. Investigation of extracellular matrix genes associated with Alzheimer’s disease in the hippocampus of experimental diabetic model rats. Int J Diabetes Dev Ctries 42, 82–90 (2022). https://doi.org/10.1007/s13410-021-00951-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-021-00951-7