Abstract
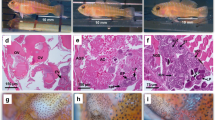
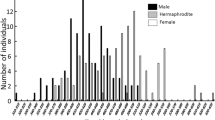
The labrid fish Pteragogus aurigarius undergoes protogynous (female-to-male) sex change, with dichromatic and dimorphic differences in sexes. Similar to some other protogynous fishes, this species is polygynous. We report the size and age at which sex change occurs by monthly sampling fishes at Tateyama, central Japan, from 2011 to 2012, and investigate conditions of sex change in rearing experiments from 2013 to 2015. Sexual phases are determined using gonad histology, and ages by otolith analysis. Of 231 sampled specimens, 99 were female, 129 were male, and three were intersexual. Of males, 86 resembled typical males, and are referred to as “normal males (NM)”, and 43 resembled females in color, which we refer to as “males with female appearance (MFA)”. NM sizes exceeded those of female, intersexual and MFA individuals. Because intersexual and MFA individuals did not differ in color or size, they may have recently transitioned to being male. Intersexual and MFA individuals ranged 85–135 mm in total length and 1–3 years in age, and appeared from September to March. Because the spawning season runs from June to September, sex change must occur afterwards. Sex change occurred in solitary females, and larger females held with other females in aquaria, both with and without aquarium partitions. Size hierarchy in social groups may be important for sex change of the largest female to male regardless of the number of individuals present.





Similar content being viewed by others
References
Endo S, Tomatsu S, Sunobe T (2020) Habitat of Pteragogus aurigarius (Labridae) in spawning and non-spawning seasons at Banda Beach, Tateyama Bay, Japan. J Tokyo Univ Mar Sci Technol 16:1–3
Ghiselin MT (1969) The evolution of hermaphroditism among animals. Q Rev Biol 44:189–208
Kobayashi K, Suzuki K, Shiobara Y (1993) Studies on the gonadal sex succession in Parapercis snyderi cross examined in captivity. Bull Inst Oceanic Res Develop, Tokai Univ 14:83–91
Kuwamura T, Nakashima Y (1998) New aspects of sex change among reef fishes: recent studies in Japan. Environ Biol Fishes 52:125–135
Kuwamura T, Suzuki S, Tanaka N, Ouchi E, Karino K, Nakashima Y (2007) Sex change of primary males in a diandric labrid Halichoeres trimaculatus: coexistence of protandry and protogyny within a species. J Fish Biol 70:1898–1906
Kuwamura T, Sunobe T, Sakai Y, Kadota T, Sawada K (2020) Hermaphroditism in fishes: an annotated list of species, phylogeny, and mating system. Ichthyol Res 67:341–360
Moyer JT (1991) Comparative mating strategies of labrid fishes. Monograph, no. 1. The Watanabe Ichthyological Institute, Tokyo
Munday PL, Buston PM, Warner RR (2006) Diversity and flexibility of sex-change strategies in animals. Trends Ecol Evol 21:89–95
Munday PL, Ryen CA, McCormick MI, Walker SPW (2009) Growth acceleration, behaviour and otolith check marks associated with sex change in the wrasse Halichoeres miniatus. Coral Reefs 28:623–634
Muñoz RC, Warner RR (2003) Alternative contexts of sex change with social control in the bucktooth parrotfish, Sparisoma radians. Environ Biol Fishes 68:307–319
Muñoz RC, Warner RR (2004) Testing a new version of the size-advantage hypothesis for sex change: sperm competition and size-skew effects in the bucktooth parrotfish, Sparisoma radians. Behav Ecol 15:129–136
Nakazono A (1979) Studies on the sex reversal and spawning behavior of five species of Japanese labrid fishes. Rep Fish Res Lab, Kyushu Univ 4:1–64
Nemtzov SC (1985) Social control of sex change in the Red Sea razorfish Xyrichtys pentadactylus (Teleostei, Labridae). Environ Biol Fishes 14:199–211
Robertson DR (1972) Social control of sex reversal in a coral reef fish. Science 177:1007–1009
Ross RM, Losey GS, Diamond M (1983) Sex change in a coral-reef fish: dependence of stimulation and inhibition on relative size. Science 221:574–575
Ross RM, Hourigan TF, Lutnesky MMF, Singh I (1990) Multiple sex changes in social group of a coral-reef fish. Copeia 1990:427–433
Sakai Y, Karino K, Nakashima Y, Kuwamura T (2002) Status-dependent behavioural sex change in a polygynous coral reef fish, Halichoeres melanonurus. J Ethol 20:101–105
Shimada K (2013) Labridae. In: Nakabo T (ed) Fishes of Japan with pictorial keys to the species third edition. Tokai University Press, Hadano-shi, Kanagawa, pp 1088–1136
Shimizu S (2016) Diversity of mating behaviour and sex change of Pteragogus aurigarius (Labridae) in Tateyama Bay, central Japan. Doctoral Dissertation, Tokyo University of Marine Science and Technology
Shimizu S, Endo S, Sasaki M, Murase A, Masuko M, Miyazawa M, Sunobe T (2016) Mating system and group spawning in the wrasse Pteragogus aurigarius in Tateyama, central Japan. Coast Ecosys 3:38–49
Victor BC (1987) The mating system of the Caribbean rosy razorfish, Xyrichtys martinicensis. Bull Mar Sci 40:152–160
Warner RR (1975) The adaptive significance of sequential hermaphroditism in animals. Am Nat 109:61–82
Warner RR (1984) Mating behavior and hermaphroditism in coral reef fishes. Am Sci 72:128–136
Warner RR, Hoffman SG (1980) Local population size as a determinant of mating system and sexual composition in two tropical marine fishes (Thalassoma spp.). Evolution 34:508–518
Warner RR, Robertson DR (1978) Sexual patterns in the 1abroid fishes of the Western Caribbean, I: the wrasses (Labridae). Smithson Cont Zool 254: 1–27
Warner RR, Swearer SE (1991) Social control of sex change in the bluehead wrasse, Thlassoma bifasciatum (Pisces: Labridae). Biol Bull 181:199–204
Acknowledgements
We are grateful to A Murase, S Sakaida, S Tomatsu, M Sasaki, Y Yokokawa and all other members of the Laboratory of Fish Behavioral Ecology for their assistance throughout this study. We thank S O’Shea from Edanz Group (https://en-author-services.edanzgroup.com/ac) for editing a draft of this manuscript. This study was supported by JSPS KAKENHI Grants to T Sunobe (no. 16K07507) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Shimizu, S., Endo, S., Kihara, S. et al. Size, age, and social control of protogynous sex change in the labrid fish Pteragogus aurigarius. Ichthyol Res 69, 75–81 (2022). https://doi.org/10.1007/s10228-021-00815-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-021-00815-4