Abstract
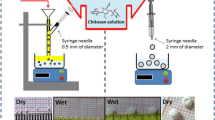
At present, water pollution becomes a very significant environmental issue. Heavy metals and organic dyes present at higher concentrations in water are dangerous for aquatic life and humanity. Chitosan nanocomposites have been used for the adsorptive removal of heavy metals and dyes due to their improved chemical activity. This paper encompassed the synthesis of chitosan-nSiO2 nanocomposites and was used to examine Cu(II) ion removal capability. The chitosan/nSiO2 nanocomposite (CSNC) adsorbents were synthesized with different weight ratios. The nanocomposites are characterized by using SEM, EDX, BET apparatus, FTIR, XRD, and TGA. The experiment is performed in batch mode by varying the operating parameters like pH, contact time, temperature, adsorbent dosage, and initial metal ion concentration. The optimum pH is 6.5 for all adsorbents. Different kinetic and isotherm models are tried. The removal efficiency was greater than 98% for the adsorbent CSNC2–1. The pseudo-2nd-order model described the kinetic process better than other models. Equilibrium data fit the best in Fritz and Schluender (IV) isotherm model for the adsorbents CWS and CSNC2–1 and Vieth and Sladek isotherm model CSNC1–1. The adsorption process was spontaneous and endothermic. Industrial effluent is also tested successfully. The application of statistical and GA modeling has also been performed successfully.
















Similar content being viewed by others
Abbreviations
- A FS :
-
Fritz–Schlunder (IV) model parameter
- a K :
-
Khan model exponent
- A KC :
-
Koble–Corrigan isotherm constant
- a R :
-
Redlich-Peterson isotherm constant [L mg−1]
- A T :
-
Tempkin isotherm equilibrium binding constant (L g−1)
- B FS :
-
Fritz–Schlunder (IV) model parameter
- b K :
-
Khan constant
- B KC :
-
Koble–Corrigan isotherm constant
- b T :
-
Tempkin isotherm constant
- B VS :
-
Vieth–Sladek isotherm constant
- b 0 :
-
Baudu isotherm equilibrium constant
- C :
-
Thickness of the boundary layer (mm)
- Ca :
-
Concentration of the adsorbate on adsorbent at equilibrium (mg L−1)
- Ce :
-
Concentration of the adsorbate at equilibrium (mg L−1)
- C t :
-
Concentration of the adsorbate at time t (mg L−1)
- C 0 :
-
Initial concentration of the adsorbate (mg L−1)
- D e :
-
Effective diffusion coefficient of the adsorbate (m2 s−1)
- E :
-
Mean free energy (kJ mol−1)
- E a :
-
Activation energy of adsorption (kJ mol−1)
- F(t) :
-
The ratio of the amount of adsorbate adsorbed per unit quantity of adsorbent at specific time t and that at the equilibrium time
- ∆G 0 :
-
Change in Gibbs free energy (kJ mol−1)
- ∆H 0 :
-
Change in enthalpy (kJ mol−1)
- K ad :
-
Dubinin–Radushkevich isotherm constant (mol2 (kJ2)−1)
- K bq :
-
Mass transfer constant (L gm−1)
- K BS :
-
Brouers–Sotolongo equilibrium constant
- \( {K}_C^0 \) :
-
Thermodynamic equilibrium constant
- \( {K}_C^{\hbox{'}} \) :
-
Apparent equilibrium constant
- KD :
-
Hill equilibrium constant
- K F :
-
Freundlich constant
- K FS :
-
Fritz-Schlunder(IIII) equilibrium constant [L mg−1]
- K J :
-
Jovanovich constant [L mg−1]
- K L :
-
Langmuir isotherm constant (L mg−1)
- k p :
-
Intraparticle diffusion rate constant (mg g−1 min−1/2)
- K RP :
-
Redlich-Peterson isotherm constant [L g−1/2]
- K RPI :
-
Radke–Prausnitz-I equilibrium constant
- K RPII :
-
Radke–Prausnitz-II equilibrium constant
- K RPIII :
-
Radke–Prausnitz-III equilibrium constant
- K S :
-
Sips equilibrium constant (L mg−1)
- K T :
-
Toth equilibrium constant
- K VS :
-
Vieth–Sladek equilibrium constant
- K 1 :
-
Fritz–Schlunder (V) equation parameter
- k 1 :
-
Pseudo-first-order rate constant (min−1)
- K 2 :
-
Fritz–Schlunder (V) equation parameter
- k 2 :
-
Pseudo-second-order rate constant (g mg−1 min−1)
- M :
-
Mass of the adsorbent per unit volume (g L−1)
- m :
-
Mass of adsorbent (g)
- m FS :
-
Fritz-Schlunder (III) model exponent
- m RPI :
-
Radke-Prausnitz-I model exponent
- m RPII :
-
Radke-Prausnitz-II model exponent
- m RPIII :
-
Radke-Prausnitz-III model exponent
- m T :
-
Toth model exponent
- m 1 :
-
Fritz–Schlunder (V) equation exponent
- m 2 :
-
Fritz–Schlunder (V) equation exponent
- n F :
-
Freundlich constant
- n H :
-
Hill co-operativity coefficient of the binding interaction
- n KC :
-
Koble–Corrigan model exponent
- n S :
-
Sips model exponent
- P 1 :
-
Weber–van Vliet model constant
- P 2 :
-
Weber-van Vliet model exponent
- P 3 :
-
Weber-van Vliet model exponent
- P 4 :
-
Weber-van Vliet model exponent
- q e :
-
Amount of metal ions adsorbed at equilibrium (mg g−1)
- q K :
-
Khan theoretical isotherm saturation capacity (mg g−1)
- q L :
-
Langmuir maximum adsorption capacity (mg g−1)
- \( {q}_{m_{BS}} \) :
-
Brouers–Sotolongo maximum adsorption capacity (mg g−1)
- \( {q}_{m_{FS}} \) :
-
Fritz-Schlunder (III) maximum adsorption capacity (mg g−1)
- \( {q}_{m_{FS5}} \) :
-
Fritz-Schlunder (V) maximum adsorption capacity (mg g−1)
- q mJ :
-
Jovanovich maximum adsorption capacity (mg g−1)
- \( {q}_{m_{RPI}} \) :
-
Radke–Prausnitz-I maximum adsorption capacity (mg g−1)
- \( {q}_{m_{RPII}} \) :
-
Radke–Prausnitz-II maximum adsorption capacity (mg g−1)
- \( {q}_{m_{RPIII}} \) :
-
Radke–Prausnitz-III maximum adsorption capacity (mg g−1)
- \( {q}_{m_T} \) :
-
Toth maximum adsorption capacity (mg g−1)
- \( {q}_{m_{VS}} \) :
-
Vieth-Sladek maximum adsorption capacity (mg g−1)
- q m0 :
-
Baudu maximum adsorption capacity (mg g−1)
- qs :
-
Theoretical isotherm saturation capacity (mg g−1)
- Q S :
-
Sips maximum adsorption capacity (mg g−1)
- \( {q}_{S_H} \) :
-
Hill theoretical isotherm saturation capacity (mg g−1)
- q t :
-
Amount of solutes adsorbed on adsorbent (mg g−1) at time t
- R :
-
Universal gas constant (J mol−1 K−1)
- R a :
-
Radius of adsorbate particles (m)
- R 2 :
-
Correlation coefficient
- S S :
-
External surface area per unit volume (m−1)
- S * :
-
Striking probability
- ∆S 0 :
-
Change in entropy (kJ mol−1)
- t :
-
Time (min)
- T :
-
Absolute temperature (K)
- V :
-
Volume of the adsorbate (L)
- x 0 :
-
Baudu isotherm parameter
- y 0 :
-
Baudu isotherm parameter
- α BS :
-
Brouers–Sotolongo exponent
- α E :
-
Initial adsorption rate in Elovich equation (mg g−1 min−1)
- α FS :
-
Fritz–Schlunder (IV) model exponent
- β :
-
Mass transfer coefficient (cm s−1)
- β E :
-
Elovich adsorption constant (g mg −1 )
- β FS :
-
Fritz–Schlunder (IV) model exponent
- β RP :
-
Exponent in Redlich-Peterson model
- θ :
-
Surface coverage
References
Ahmed, M. A., Abdelbar, N. M., & Mohamed, A. A. (2018). Molecular imprinted chitosan-TiO2 nanocomposite for the selective removal of Rose Bengal from wastewater. International Journal of Biological Macromolecules, 107, 1046–1053.
Alencar, W. S., Acayanka, E., Lima, E. C., Royer, B., de Souza, F. E., Lameira, J., & Alves, C. N. (2012). Application of Mangiferaindica (mango) seeds as a biosorbent for removal of Victazol Orange 3R dye from aqueous solution and study of the biosorption mechanism. Chemical Engineer Journal, 209, 577–588.
Alsabagh, A. M., Fathy, M., & Morsi, R. E. (2015). Preparation and characterization of chitosan/silver nanoparticle/copper nanoparticle/carbon nanotube multifunctional nano-composite for water treatment: Heavy metals removal; kinetics, isotherms and competitive studies. RSC Adv, 5, 55774–55783.
Alves, N. M., & Mano, J. F. (2008). Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications. Int J Biol Macromol, 43, 401–414.
APHA - Standard Methods for Examination of Water and Wastewater (1998) 20th ed., APHA, AWWA, WEF, Washington, DC, New York. .
Asandei, D., Bulgariu, L., & Bobu, E. (2009). Lead (II) removal from aqueous solutions by adsorption onto chitosan. Cerllulose Chem Technol, 43(4–6), 211–216.
Awual, M. R., Hasan, M. M., Khaleque, M. A. & Sheikh, M. C. (2016). Treatment of copper(II) containing wastewater by a newly developed ligand based facial conjugate materials. Chemical Engineer Journal, 288, 368–376.
Banerjee, M., Bar, N., Basu, R. K., & Das, S. K. (2017). Comparative study of adsorptive removal of Cr(VI) ion from aqueous solution in fixed bed column by peanut shell and almond shell using empirical models and ANN. Environ Sci Poll Res, 24, 10604–10620.
Banerjee, M., Basu R. K., & Das, S. K. (2019a). Cu(II) removal using green adsorbents: Kinetic modeling and plant scale-up design. Environmental Science and Pollution Research, 26(12), 11542–11557.
Banerjee, M., Basu, R. K., & Das, S. K. (2019b). Adsorptive removal of Cu(II) by pistachio shell: Isotherm study, kinetic modelling and scale-up designing—Continuous mode. Environ Technol Innov, 15, 100419.
Bar, N. & Das, S. K. (2012). Frictional pressure drop for gas—Non-Newtonian liquid flow through 90o & 135o circular bend: Prediction using empirical correlation & ANN. International Journal of Fluid Mechanics Research, 39(5), 416–437.
Bar, N., & Das, S. K. (2020). Applicability of ANN in adsorptive removal of Cd(II) from aqueous solution. In Waste Management, Concepts, Methodologies, Tools and Applications, Ed. Information Resources Management Association, IGI Global, Hershey, USA, Chapter, 67, 1453–1491.
Baudu, M. (1990). Etude des interactions solute-fibres de charbonactif: Application et regeneration, Ph.D. Thesis, Université de Rennes I.
Boyd, G. E., Adamson, A. W. & Mayers, Jr L. S. (1947). The exchange adsorption of ions from aqueous solutions on organic zeolites: II kinetics. Journal of the American Chemical Society, 69, 2836–2848.
Beppu, M. M., Arruda, E. J., Vieira, R. S., & Santos, N. N. (2004). Adsorption of Cu(II) on porous chitosan membranes functionalized with histidine. J Membr Sci, 240, 227–235.
Brouers, F., Sotolongo, O., Marquez, F., & Pirard, J. P. (2005). Microporous and heterogeneous surface adsorption isotherms. Physica, A349, 271–282.
Chan, P., Ng, S., Seng, C., & Lim, P. (2012). Removal of Cu(II) from aqueous solutions by living and non-living cultured sludges: Equilibrium modelling. Int J Environ Technol Manag, 15, 79–93.
Chen, H., Dai, G. L., Zhao, J., Zhong, A. G., Wu, J. Y., & Yan, H. (2010). Removal of copper(II) ions by a biosorbent—Cinnamomumcamphora leaves powder. J Hazard Mater, 177, 228–236.
Chen, H., Lin, J., Zhang, N., Chen, L., Zhong, S., Zhang, W., Ling, Q., & Wang, Y. (2018). Preparation of MgAl-EDTA-LDH based electrospunnanofiber membrane and its adsorption properties of copper(II) from wastewater. Journal of Hazardous Materials, 345, 1–9.
Cheng, Z., Liu, X., Han, M., & Ma, W. (2010). Adsorption kinetic character of copper ions onto a modified chitosan transparent thin membrane from aqueous solution. J Hazard Mater, 182, 408–415.
Das, A., Bar, N., & Das, S. K. (2020). Pb(II) adsorption from aqueous solution by nutshells, green adsorbent: Adsorption studies, regeneration studies, scale-up design, its effect on biological indicator and MLR modelling. J Colloid Interf Sci, 580, 245–255.
Dakiky, M., Khamis, M., Manassra, A., & Mer’eb, M. (2002). Selective adsorption of chromiumVI in industrial wastewater using low-cost abundantly available adsorbents. Adv Environ Res, 6, 533–540.
Dlugosz, O., & Banach, M. (2018). Kinetic, isotherm and thermodynamic investigations of the adsorption of Ag+ and Cu2+ on vermiculite. J Mol Liquids, 258, 295–309.
Do, D. D. (1998). Adsorption analysis: Equilibria and kinetics. London: Imperial College Press.
Dubinin, M. M., Zaverina, E. D., & Radushkevich, L. V. (1947). Sorption and structure of active carbons I, Adsorption of organic vapors. Zhurnal Fizicheskoi Khimii, 21, 1351–1362.
Elsoud, M. M. A., & Kady, E. M. El. (2019). Current trends in fungal biosynthesis of chitin and chitosan. Bulletin of the National Research Centre, 43(56). https://doi.org/10.1186/s42269-019-0105-y.
Elwakeel, K. Z. (2010). Environmental application of chitosan resins for the treatment of water and wastewater. J Disper Sci Technol, 31(3), 273–288.
EPR-Environmental Protection Rules, (Schedule VI) of Central Pollution Control Board, Govt. of India (1986). Available: http://cpcb.nic.in/GeneralStandards.pdf. Accessed 10 Mar 2020.
Fan, H. L., Zhou, S. F., Jiao, W. Z., Qi, G. S., & Liu, Y. Z. (2017). Removal of heavy metal ions by magnetic chitosan nanoparticles prepared continuously via high-gravity reactive precipitation method. Carbohydr Polym, 174, 1192–1200.
Freundlich, H. M. F. (1906). Over the adsorption in solution. J Phys Chem, 57, 385–471.
Fritz, W., & Schlunder, E. U. (1974). Simultaneous adsorption equilibria of organic solutes in dilute aqueous solution on activated carbon. Chemical Engineering Science, 29, 1279–1282.
Himmelblau, D. M. (2000). Applications of artificial neural networks in chemical engineering. Korean Journal of Chemical Engineering, 17(4), 373–392.
Himmelblau, D. M. (2008). Accounts of experiences in the application of artificial neural networks in chemical engineering. Ind Eng Chem Res, 47, 5782–5796.
Ho, Y. S. (2004). Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics, 59(1), 171–177.
Ho, Y. S., & McKay, G. (2000). The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res, 34(3), 735–742.
Ho, Y. S., & McKay, G. (2002). Application of kinetic models to the sorption of copper(II) on to peat. Ads Sci Technol, 20(8), 797–815.
Ho, Y. S., McKay, G., Wase, D. A. J., & Foster, C. F. (2000). Study of the sorption of divalent metal on peat. Ads Sci Technol, 18(7), 639–650.
Hill, T. L. (1946). Statistical mechanics of multi molecular adsorption II. Localized and mobile adsorption and absorption. J Chem Phys, 14(7), 441–453.
Hsieh, S. H., Horng, J. J., & Tsai, C. K. (2006). Growth of carbon nanotube on micro-sized Al2O3 particle and its application to adsorption of metal ions. Journal of Materials Research, 21, 1269–1273.
IS 10500: 2012. Indian Standards for Drinking water (IS10500:2012) Available on https://law.resource.org/pub/in/bis/S06/is.10500.2012.pdf. Accessed 10 Mar 2020.
Jovanovich, D. S. (1969). Physical adsorption of gases, I: Isotherms for monolayer and multilayer adsorption. Colloid & Polymer Science, 235, 1203–1214.
Khan, A. R., Al-Waheab, I. R., & Al-Haddad, A. A. (1996). Generalized equation for adsorption isotherms for multicomponent organic pollutants in dilute aqueous solution. Environ Techno, l17, 13–23.
Koble, R. A., & Corrigan, T. E. (1952). Adsorption isotherms for pure hydrocarbons. Ind Eng Chem, 44, 383–387.
Kyzas, G. Z., & Bikiaris, D. N. (2015). Recent modifications of chitosan for adsorption applications: A critical and systematic review. Marine Drugs, 13, 312–337.
Lagergren, S. (1898). Zurtheorie der sogenannten adsorption gelösterstoffle, Kungliga Sevenska Vetenskapasakademiens. Handilingar, 24, 1–39.
Langmuir, I. (1918). The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc, 40(9), 1361–1368.
Lasheen, M. R., El-Sherif, I. Y., Tawfik, M. E., El-Wakeel, S. T., & El-Shahat, M. F. (2016). Preparation and adsorption properties of nano magnetite chitosan films for heavy metal ions from aqueous solution. Mater Res Bull, 80, 344–350.
Li, J., Jiang, B., Liu, Y., Qiu, C., Hu, J., Qian, G., Guo, W., & Ngo, H. (2017). Preparation and adsorption properties of magnetic chitosan composite adsorbent for Cu2+ removal. Journal of Cleaner Production, 158, 51–58.
Linder, M. C., & Goode, C. A. (1991). In E. Frieden (Ed.), Biochemistry of the Elements (pp. 331–342). New York: Plenum Press.
Liu, X., Hu, Q., Fang, Z., Zhang, X., & Zhang, B. (2009). Magnetic chitosan nanocomposites: A useful recyclable tool for heavy metal ion removal. Langmuir, 25, 3–8.
Lucaci, A. R., Bulgariu, D., Popescu, M.-C., & Bulgariu, L. (2020). Adsorption of Cu(II) ions on adsorbent materials obtained from marine red algae Callithamnion corymbosum sp. Water, 12, 372.
Maiti, S. B., Bar, N., & Das, S. K. (2019). Fluidization using pseudoplastic liquids—Elutriation and ANN modeling. Adv Powder Technol, 30(12), 2940–2946.
Maiti, S. B., Bar, N., & Das, S. K. (2020). Bed expansion in two-phase liquid-solid fluidized beds with non-Newtonian fluids and ANN modelling. Proc. Global AI Congress 2019. In Advances in Intelligent Systems and Computing 1112 (pp. 33–45). Hershey, USA: IGI Global.
Maiti, S. B., Let, S., Bar, N., & Das, S. K. (2018). Non-spherical solid-non-Newtonian liquid fluidization and ANN modelling: Minimum fluidization velocity. Chem Eng Sci, 176, 233–241.
Mitra, T., & Das, S. K. (2020). Removal of Cu(II) ions using bio-adsorbents in fixed—Bed continuous bed mode—A comparative study and scale-up design. Environ Prog Sus Energy, 39(5), e 013417.
Monier, M., Ayad, D. M., Wei, Y., & Sarhan, A. A. (2010). Preparation and characterization of magnetic chelating resin based on chitosan for adsorption of Cu (II) ions, Co (II) ions, and Ni (II) ions. React Funct Polym, 70, 257–266.
Mozurkewich, M., & Benso, S. W. (1984). Negative activation energies and curved Arrhenius plots. 1. Theory of reactions over potential wells. J Phys Chem, 88(25), 6429–6435.
Muzzarelli, R. A. (1973). Natural chelating polymers. New York: Pergamon Press.
Nag, S., Mondal, A., Bar, N., & Das, S. K. (2017). Biosorption of chromium(VI) from aqueous solutions and ANN modelling. Environ Sci Pollut Res, 24(23), 18817–18835.
Nag, S., Bar, N., & Das, S. K. (2020). Cr(VI) removal from aqueous solution using green adsorbents in continuous bed column—Statistical and GA-ANN hybrid modelling. Chem Eng Sci, 226(23), 115904.
Naiya, T. K., Bhattacharya, A. K., & Das, S. K. (2009). Adsorption of Cd(II) and Pb(II) from aqueous solutions on activated alumina. J Colloid Interf Sci, 333, 14–26.
Nithya, R., Gomathi, T., Sudha, P. N., Venkatesan, J., Anil, S., & Kim, S. K. (2016). Removal of Cr(VI) from aqueous solution using chitosan-g-poly(butylacrylate)/silica gel nanocomposite. Int J Biol Macromol, 87, 545–554.
Pirdashti, M., Curteanu, S., Kamangar, M. H., Hassim, M. H., & Khatami, M. A. (2013). Artificial neural networks: Applications in chemical engineering. Rev Chem Eng, 29(4), 205–239.
Reddi, M. R. G., Gomathi, T., Saranya, M., & Sudha, P. N. (2017). Adsorption and kinetic studies on the removal of chromium and copper onto Chitosan-g-maliec anhydride-g-ethylene dimethacrylate. Int J Biol Macromol, 104(Pt B), 1578–1585.
Rinaudo, M. (2006). Chitin and chitosan: Properties and applications. Prog Poly Sci, 31, 603–632.
Reis, L. G. T. D., Robaina, N. F., Pacheco, W. F., & Cassella, R. J. (2011). Separation of malachite green and methyl green cationic dyes from aqueous medium by adsorption on amberlite XAD-2 and XAD-4 resins using sodium dodecylsulfate as carrier. Chemical Engineer Journal, 171, 532–540.
Redlich, O., & Peterson, D. L. (1959). A useful adsorption isotherm. J Phy Chem, 63(6), 1024–1026.
Saad, A. H. A., Azzam, A. M., El-Wakeel, S. T., Mostafa, B. B., & El-latif, M. B. A. (2018). Removal of toxic metal ions from wastewater using ZnO@Chitosancoreshellnanocomposite. Environ Nanotechnol Monit Manag, 9, 67–75.
Salam, M. A., Makki, M. S. I., & Abdelaal, M. Y. A. (2011). Preparation and characterization of multi-walled carbon nanotubes/chitosan nanocomposite and its application for the removal of heavy metals from aqueous solution. Journal of Alloys and Compounds, 509, 2582–2587.
Sarkar, S., & Das, S. K. (2016). Removal of Cr(VI) & Cu(II) ions from aqueous solution by rice husk. Desalin Water Treat, 57(43), 20340–20349.
Sarkar, P., Moyez, S. A., Dey, A., Roy, S., & Das, S. K. (2017). Experimental investigation of photocatalytic and photovoltaic activity of titania/rice husk crystalline nano-silica hybrid composite. Solar Energy Materials & Solar Cells, 172, 93–98.
Soltani, R. D. C., Khataee, A. R., & Safari, M. (2013). Joo SW, Preparation of bio-silica/chitosan nanocomposite for adsorption of a textile dye in aqueous solutions. International Biodeterioration and Biodegradation, 85, 383–391.
Srivastava, V. C., Mall, I. D., & Mishra, I. M. (2006). Characterization of mesoporous rice husk ash (RHA) and adsorption kinetics of metal ions from aqueous solution onto RHA. J Hazard Mater, B134, 257–267.
Singha, B., & Das, S. K. (2013). Adsorptive removal of Cu(II) from aqueous solution & industrial effluent using natural/agricultural wastes. Coll Surf B: Biointer, 107, 97–108.
Sips, R. (1948). Combined form of Langmuir and Freundlich equations. J Chem Phys, 16, 490–495.
Steenkamp, G. C., Keizer, K., Neomagus, H. W. J. P., & Krieg, H. M. (2002). Copper (II) removal from polluted water with alumina/chitosan composite membranes. J Mem Sci, 197, 147–156.
Tekin, K., Uzun, L., Sahin, C. A., Bektas, S., & Denizli, A. (2011). Preparation and characterization of composite cryogels containing imidazole group and use in heavy metal removal. React Funct Polym, 71, 985–993.
Temkin, M. I. & Pyzhev, V. (1940). Kinetics of ammonia synthesis on promoted iron catalyst. Acta Physicochim USSR, 12, 327–356.
Toth, J. (2000). Calculation of the BET-compatible surface area from any type I isotherms measured above the critical temperature, Journal of Colloid and Interface Science, 225, 378–383.
van Vliet, B. M., Weber, W. J. & Hozumi, H. (1980). Modeling and prediction of specific compound adsorption by activated carbon and synthetic adsorbents. Water Research, 14, 1719–1728.
Voskoboinik, I., Mar, J., Strausak, D., & Camakaris, J. (2001). The regulation of catalytic activity of the menkes copper-translocating p-type ATPase role of high affinity copper-binding sites. J Biological Chem, 276(30), –28620, 28627.
Wan, M. W., Kan, C. C., Rogel, B. D., & Dalid, M. L. P. (2010). Adsorption of copper (II) and lead (II) ions from aqueous solution on chitosan-coated sand. Carbohydr Polym, (80), 891–899.
Wang, L. Y. & Wang, M. J. (2016). Removal of heavy metal ions by poly(vinyl alcohol) and carboxymethyl cellulose composite hydrogels prepared by a freeze–thaw method. ACS Sustainable Chemistry & Engineering, 4, 2830–2837.
Wang, Y. J., Jia, D. A., Sun, R. J., Zhu, H. W., & Zhou, D. M. (2008). Adsorption and cosorption of tetracycline and copper(II) on montmorillonite as affected by solution pH. Environ Sci Technol, 42, 3254–3259.
Weber, W. J., & Moris, J. C. (1963). Kinetics of adsorption on carbon from solution. J San Eng. Div, 89(2), 31–60.
Wu, N., Wei, H., & Zhang, L. (2012). Efficient removal of heavy metal ions with biopolymer template synthesized mesoporoustitania beads of hundreds of micrometers size. Environmental Science & Technology, 46, 419–425.
Wu, Y. J., Zhang, L. J., Gao, C. L., Ma, J. Y., Ma, X. H., & Han, R. P. (2009). Adsorption of copper ions and methylene blue in a single and binary system on wheat straw. Journal of Chemical & Engineering Data, 54(12), 3229–3234.
Yu, B., Zhang, Y., Shukla, A., Shukla, S. S., & Dorris, K. I. (2000). The removal of heavy metal from aqueous solution by sawdust adsorption-removal of copper. J Hazard Mater, 80, 33–42.
Zavareh, S., Zarei, M., Darvishi, F., & Azizi, H. (2015). As(III) adsorption and antimicrobial properties of Cu–chitosan/alumina nanocomposite. Chemical Engineer Journal, 273, 610–621.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 63.4 kb)
Rights and permissions
About this article
Cite this article
Bhattacharya, S., Bar, N., Rajbansi, B. et al. Adsorptive Elimination of Cu(II) from Aqueous Solution by Chitosan-nanoSiO2 Nanocomposite—Adsorption Study, MLR, and GA Modeling. Water Air Soil Pollut 232, 161 (2021). https://doi.org/10.1007/s11270-021-05070-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-05070-x