Abstract
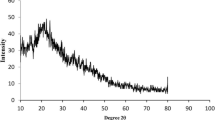
In the present work, we focused on extracting, separating, formulating, and, finally, characterizing quercetin. Chitosan/GMO nanoparticles were investigated to controlled release for targeting colonic region. Quercetin is an active biomolecule isolated from peels of pomegranate fruit, separated by different chromatographic techniques, and formulated into nanoformulation to bring it to increase its aqueous solubility. Nanoparticles were prepared by using chitosan, glyceryl monooleate (GMO), and poloxamer 407 using probe sonicator and high-pressure homogenization method. Characterization of nanoparticles was carried out by particle size, zeta potential, differential scanning colorimetry (DSC), X-ray diffraction (XRD), scanning electron microscope (SEM), entrapment efficiency, loading content, in vitro release, and stability study. They showed approximately 78.82% encapsulation with an average size of 145.5 ± 0.66 nm and zeta potential + 14.7 mV. The cumulative in vitro drug release up to 24 h at 77.16% was achieved suggesting towards efficacy of green synthesized chitosan nanoparticles for colonic delivery applications. From all our findings, it can be concluded that work will facilitate the extraction, design, and fabrication of nanoparticles for the protection and sustained release of quercetin biomolecule, particularly to the colonic region. The release performance of chitosan/GMO nanoparticles loaded with quercetin at different pH conditions was greatly affected by the materials used in the preparation, which allows maximum release at colonic pH. Hence, it is a unique approach for colonic delivery of drugs having appropriate site specificity and feasibility and controlled release of biomolecule quercetin.
Graphical abstract
Biomolecule quercetin isolated from peels of pomegranate fruit, then separated and characterized by Column, Flash, GC, IR, NMR, LC-MS, HPLC and HPTLC techniques. Formulation and characterization of Qu-loaded Chi nanoparticles by FTIR, XRD, DSC, SEM, Encapsulation effiency and Loading content results influencing on critical quality attributes analysed by central composite design. Optimized Qu-loaded Chi nanoparticles had a significant sustained in-vitrorelease at colonic region.











Similar content being viewed by others
Change history
26 April 2021
A Correction to this paper has been published: https://doi.org/10.1007/s12247-021-09558-1
References
Luo W, et al. Antioxidant and antiproliferative capacities of phenolics purified from Phyllanthus emblica L. fruit. Food Chem. 2011;126(1):277–282.
Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002;13(10):572–84.
Erlund I. Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutr Res. 2004; 24(10): p. 851–874.
Yao LH, et al. Flavonoids in food and their health benefits. Plant Foods Hum Nutr. 2004;59(3):113–22.
Liu X, et al. Identification of phenolics in the fruit of emblica (Phyllanthus emblica L.) and their antioxidant activities. Food Chem. 2008;109(4):909–915.
Salgado JM, et al. Increased antioxidant content in juice enriched with dried extract of pomegranate (Punica granatum) peel. Plant Foods Hum Nutr. 2012;67(1):39–43.
Lansky EP, Newman RA. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J Ethnopharmacol. 2007;109(2):177–206.
Qnais E, et al. Antidiarrheal activity of the aqueous extract of Punica granatum (Pomegranate) peels. Pharm Biol. 2007;45(9):715–720.
Rauf A, et al. Anticancer potential of quercetin: a comprehensive review. Phytother Res. 2018;32(11):2109–30.
Negi P, Jayaprakasha G, Jena B. Antioxidant and antimutagenic activities of pomegranate peel extracts. Food Chem. 2003;80(3):393–7.
Li Y, et al. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006;96(2):254–60.
Peterson LR, Dalhoff A. Towards targeted prescribing: will the cure for antimicrobial resistance be specific, directed therapy through improved diagnostic testing? J Antimicrob Chemother. 2004;53(6):902–5.
Kawai Y, et al. Macrophage as a target of quercetin glucuronides in human atherosclerotic arteries implication in the anti-atherosclerotic mechanism of dietary flavonoids. J Biol Chem. 2008;283(14):9424–34.
Liao H, et al. A bibliometric analysis and visualization of medical big data research. Sustainability. 2018;10(1):166.
Nuengchamnong N, Hermans-Lokkerbol A, Ingkaninan K. Separation and detection of the antioxidant flavonoids, rutin and quercetin, using HPLC coupled on-line with colorimetric detection of antioxidant activity. Naresuan University Journal: Science and Technology (NUJST). 2013;12(2):25–37.
Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008;585(2–3):325–37.
Wu T-H, et al. Preparation, physicochemical characterization, and antioxidant effects of quercetin nanoparticles. Int J Pharm. 2008;346(1–2):160–8.
Patra A, et al. Formulation and evaluation of mixed polymeric micelles of quercetin for treatment of breast, ovarian, and multidrug resistant cancers. Int J Nanomed. 2018;13:2869.
Ramadon D, Anwar E, Harahap Y. In vitro penetration and bioavailability of novel transdermal quercetin-loaded ethosomal gel. Indian J Pharm Sci. 2018;79(6):948–56.
Ader P, Wessmann A, Wolffram S. Bioavailability and metabolism of the flavonol quercetin in the pig. Free Radical Biol Med. 2000;28(7):1056–67.
Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf, B. 2010;75(1):1–18.
Vijayakumar A, et al. Quercetin-loaded solid lipid nanoparticle dispersion with improved physicochemical properties and cellular uptake. AAPS Pharm Sci Tech. 2017;18(3):875–83.
Sahle FF, et al. Formulation and in vitro evaluation of polymeric enteric nanoparticles as dermal carriers with pH-dependent targeting potential. Eur J Pharm Sci. 2016;92:98–109.
Kamaly N, et al. Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem Rev. 2016;116(4):2602–63.
Kumari A, et al. Development of biodegradable nanoparticles for delivery of quercetin. Colloids Surf, B. 2010;80(2):184–92.
Han Q, et al. Quercetin nanoparticles with enhanced bioavailability as multifunctional agents toward amyloid induced neurotoxicity. J Mater Chem B. 2018;6(9):1387–93.
Khor CM, et al. Preparation and characterization of quercetin/dietary fiber nanoformulations. Carbohyd Polym. 2017;161:109–17.
Dudhani AR, Kosaraju SL. Bioadhesive chitosan nanoparticles: preparation and characterization. Carbohyd Polym. 2010;81(2):243–51.
Gulbake A, Jain SK. Chitosan: a potential polymer for colon-specific drug delivery system. Expert Opin Drug Deliv. 2012;9(6):713–29.
Koffi E, et al. Effect of solvent type on extraction of polyphenols from twenty three Ivorian plants. J Anim Plant Sci (JAPS). 2010;5(3):550–8.
Stalikas CD. Extraction, separation, and detection methods for phenolic acids and flavonoids. J Sep Sci. 2007;30(18):3268–95.
Chang C-C, et al. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002. 10(3).
Altemimi A, et al. Simultaneous extraction, optimization, and analysis of flavonoids and polyphenols from peach and pumpkin extracts using a TLC-densitometric method. Chem Cent J. 2015;9(1):39.
Anandhakumar S, et al. Preparation of collagen peptide functionalized chitosan nanoparticles by ionic gelation method: an effective carrier system for encapsulation and release of doxorubicin for cancer drug delivery. Mater Sci Eng, C. 2017;70:378–85.
Olejniczak S, Potrzebowski MJ. Solid state NMR studies and density functional theory (DFT) calculations of conformers of quercetin. Org Biomol Chem. 2004;2(16):2315–22.
Sanghavi N, Srivastava R, Malode Y. Isolation and identification of the flavonoid “quercetin” from Tridax procumbens linn. Int J Pharm Sci Res. 2014;5(4):1454.
Leela V, Saraswathy A. Quantification of pharmacologically active markers gallic acid, quercetin and lupeol from acacia leucophloea wild flowers by HPTLC method. J Anal Bioanal Tech. 2013;4:2–5.
Sarabandi K, et al. Production of reconstitutable nanoliposomes loaded with flaxseed protein hydrolysates: stability and characterization. Food Hydrocolloids. 2019;96:442–50.
Pandit AA, Dash AK. Surface-modified solid lipid nanoparticulate formulation for ifosfamide: development and characterization. Nanomedicine. 2011;6(8):1397–412.
Park SN, Jo NR, Jeon SH. Chitosan-coated liposomes for enhanced skin permeation of resveratrol. J Ind Eng Chem. 2014;20(4):1481–5.
Hosseini SF, et al. Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: preparation, characterization and in vitro release study. Carbohyd Polym. 2013;95(1):50–6.
Shetta A, Kegere J, Mamdouh W. Comparative study of encapsulated peppermint and green tea essential oils in chitosan nanoparticles: encapsulation, thermal stability, in-vitro release, antioxidant and antibacterial activities. Int J Biol Macromol. 2019;126:731–42.
Kavaz D, Idris M, Onyebuchi C. Physiochemical characterization, antioxidative, anticancer cells proliferation and food pathogens antibacterial activity of chitosan nanoparticles loaded with Cyperus articulatus rhizome essential oils. Int J Biol Macromol. 2019;123:837–45.
Nguyen TX, et al. Chitosan-coated nano-liposomes for the oral delivery of berberine hydrochloride. J Mater Chem B. 2014;2(41):7149–59.
Mladenovska K, et al. 5-ASA loaded chitosan–Ca–alginate microparticles: preparation and physicochemical characterization. Int J Pharm. 2007;345(1–2):59–69.
Freitas C, Müller R. Correlation between long-term stability of solid lipid nanoparticles (SLN™) and crystallinity of the lipid phase. Eur J Pharm Biopharm. 1999;47(2):125–32.
Smitha B, Sridhar S, Khan A. Chitosan–sodium alginate polyion complexes as fuel cell membranes. Eur Polymer J. 2005;41(8):1859–66.
Yu S-H, et al. Preparation and characterization of radical and pH-responsive chitosan–gallic acid conjugate drug carriers. Carbohyd Polym. 2011;84(2):794–802.
Abdou EM, Masoud MM. Gallic acid–PAMAM and gallic acid–phospholipid conjugates, physicochemical characterization and in vivo evaluation. Pharm Dev Technol. 2018;23(1):55–66.
Venkatesan J, et al. Preparation and characterization of chitosan–carbon nanotube scaffolds for bone tissue engineering. Int J Biol Macromol. 2012;50(2):393–402.
Singh P, Verma N. Preparation and characterization of nanomicelle for ocular delivery of fluoroquinolone derivative. J Drug Deliv Ther. 2019;9(2-s):355–365.
Pralhad T, Rajendrakumar K. Study of freeze-dried quercetin–cyclodextrin binary systems by DSC, FT-IR, X-ray diffraction and SEM analysis. J Pharm Biomed Anal. 2004;34(2):333–9.
Vozza G, et al. Application of Box-Behnken experimental design for the formulation and optimisation of selenomethionine-loaded chitosan nanoparticles coated with zein for oral delivery. Int J Pharm. 2018;551(1–2):257–69.
Pasparakis G, Bouropoulos N. Swelling studies and in vitro release of verapamil from calcium alginate and calcium alginate–chitosan beads. Int J Pharm. 2006;323(1–2):34–42.
Tamilvanan S, et al. Stability assessment of injectable castor oil-based nano-sized emulsion containing cationic droplets stabilized by poloxamer–chitosan emulsifier films. AAPS Pharm Sci Tech. 2010;11(2):904–9.
Acknowledgements
The authors express their deep sense of gratitude towards Bharati Vidyapeeth College of Pharmacy (Kolhapur, Maharashtra, India) and Government College of Pharmacy (Karad, Maharashtra) for the provision of obligatory facilities to carry out present research work. The authors are profusely thankful to Dr. Riyaz Ali M. Osmani, Department of Biosciences and Bioengineering (BSBE), Indian Institute of Technology Bombay (IITB), Mumbai, for his valuable inputs and constructive suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Disclaimer
The authors alone are responsible for the content and writing of this research article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The original version of this article unfortunately contained a mistake in figure 4. Originally, figure 4 is identical to figure 9.
Rights and permissions
About this article
Cite this article
Patil, P., Killedar, S. Green Approach Towards Synthesis and Characterization of GMO/Chitosan Nanoparticles for In Vitro Release of Quercetin: Isolated from Peels of Pomegranate Fruit. J Pharm Innov 17, 764–777 (2022). https://doi.org/10.1007/s12247-021-09552-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-021-09552-7