Abstract
While urbanisation remains a major threat to biodiversity, urban areas can sometimes play an important role in protecting threatened species, especially exploited taxa such as parrots. The Hispaniolan Parakeet Psittacara chloropterus has been extirpated across much of Hispaniola, including from most protected areas, yet Santo Domingo (capital city of the Dominican Republic) has recently been found to support the island’s densest remaining population. In 2019, we used repeated transects and point-counts across 60 1 km2 squares of Santo Domingo to examine the distribution of parakeets, identify factors that might drive local presence and abundance, and investigate breeding ecology. Occupancy models indicate that parakeet presence was positively related to tree species richness across the city. N-Mixture models show parakeet encounter rates were correlated positively with species richness of trees and number of discrete ‘green’ patches (> 100 m2) within the survey squares. Hispaniolan Woodpecker Melanerpes striatus, the main tree-cavity-producing species on Hispaniola, occurs throughout the city, but few parakeet nests are known to involve the secondary use of its or other cavities in trees/palms. Most parakeet breeding (perhaps 50–100 pairs) appears to occur at two colonies in old buildings, and possibly only a small proportion of the city’s 1500+ parakeets that occupy a single roost in street trees breed in any year. Our models emphasise the importance of parks and gardens in providing feeding resources for this IUCN Vulnerable species. Hispaniola’s urban centres may be strongholds for populations of parakeets and may even represent sources for birds to recolonise formerly occupied areas on the island.
Similar content being viewed by others
Introduction
Urbanisation is a significant threat to the world’s wildlife with, for example, residential and commercial development listed as a contributor to declines in 609 Threatened/Near Threatened bird species worldwide (BirdLife International 2020). However, there is a growing appreciation that urban areas may also act as valuable habitats for wildlife generally (e.g. Alvey 2006) and for some threatened taxa in particular (Ives et al. 2016). This value may stem from the diversity of habitats within urban centres (e.g. Alberti 2005), from the protection that urban centres offer (e.g. DeStefano & DeGraaf 2003), and/or from various anthropogenic food resources (e.g. Stofberg et al. 2019). A group of birds that has adapted particularly well to urban living are parrots, for which cities represent fruitful places for exotic species to establish (Butler 2005) and havens for populations of otherwise rare species to persist or even thrive (Irumba et al. 2016). Several extrinsic factors may allow parrots above other birds to thrive in cities: large green spaces which, to some extent, mimic forest patches (Davis et al. 2012) and between which parrots are naturally selected to travel; buildings which mimic natural nest-sites (Tella et al. 2014); the widespread availability of fruiting trees (Clergeau & Vergnes 2011); high cognitive ability and behavioural plasticity for discriminating and taking advantage of opportunities (Salinas-Melgoza et al. 2013, Sol et al. 2013); gregarious behaviour for the rapid assimilation of information (Chapman et al. 1989); and the protection from trapping by well-enforced laws, the ubiquity of potential crime witnesses, and/or urban dwellers having little interest in trapping parrots (Martens & Woog 2017).
The Hispaniolan Parakeet Psittacara chloropterus is a threatened parrot (IUCN status Vulnerable) endemic to the Caribbean island of Hispaniola (Haiti and the Dominican Republic [hereafter DR]) (del Hoyo & Collar 2014). Hispaniolan Parakeets are obligate cavity nesters and, as with other secondary cavity nesters on the island (e.g. Hispaniolan Amazon Amazona ventralis and Hispaniolan Trogon Priotelus roseigaster), are thought to rely on cavities produced by the endemic Hispaniolan Woodpecker Melanerpes striatus (Latta et al. 2006). Hispaniolan Parakeets are gregarious birds that feed on fruit and seeds from a wide variety of native and non-native species (Olson 2015), but their habitat needs and breeding ecology are little known (Collar et al. 2020).
A review of records up to 1930 (Wetmore & Swales 1931) suggests that the species was once widespread and numerous on the island, but became rarer in lower coastal areas in the later nineteenth century, although ‘abundant at certain seasons’ (presumably because it descended in winter from montane areas where it maintained its numbers); around 1870, flocks could be so great that hundreds were shot in defence of maize crops, and even in 1927 ‘thousands were seen in small flocks daily’ in and west of San Juan de la Maguana, DR. A more recent review of records up to the year 2000 (Keith et al. 2003) provided further evidence of decline, describing the species as ‘extirpated or uncommon in most areas’ and citing ‘at least a 95% decline in Los Haitises National Park, DR from 1976 to 1996’ (a site from which it was soon afterwards declared extirpated; Latta et al. 2006). The parakeet has declined and locally disappeared from the landscape across Hispaniola (Wiley 1991, Dod 1992, Collar et al. 1994, Snyder et al. 2000, Stattersfield & Capper 2000, Kirwan et al. 2019). Notably, however, instead of retreating from the centres of human activity into the most remote areas (the typical pattern of decline in species) the parakeet possesses a relatively strong presence in Santo Domingo, DR’s capital, and Santiago de los Caballeros, DR’s ‘second’ city (Luna et al. 2018).
The decline of the parakeet has been attributed to habitat loss, persecution as a crop pest, hunting for food, and exploitation as a pet (Wiley 1991, Collar et al. 1994, Stattersfield & Capper 2000, Keith et al. 2003, Luna et al. 2018), but the relative contribution of these threats to the decline is unknown. However, if foraging opportunities, shelter and nest-sites are key components of the parakeet’s habitat, Santo Domingo may have been or become a bulwark against at least two of these threats. The city certainly appears now to support the largest/densest single population of the Hispaniolan Parakeet on the planet. As a first step in seeking to understand, and in future to manage, the factors influencing this important population, we examined the distribution of parakeets across Santo Domingo and related this to characteristics of the city’s green spaces. We also report on the parakeet’s urban roosting, and known nesting sites, and relate its distribution to that of the cavity-producing Hispaniolan Woodpecker.
Methods
Survey area and survey methods
The Greater Santo Domingo area (18°28′N 69°53′W) is the largest urban centre in the DR, supporting a population of 3.2 million people in an area of 104 km2 (Population Stat 2019). This coastal metropolitan area is usually defined as the Distrito Nacional (the capital city and seat of government) plus eight municipalities from the surrounding Santo Domingo and San Cristóbal Provinces. Records on eBird suggest that parakeets are most regularly recorded in the Distrito Nacional. Although the species is recorded often, the spatial distribution of these records is not sufficient to investigate habitat use; we therefore designed a survey to cover the city more widely. It is possible that breeding and feeding sites exist outside of our survey areas; however, this area covers the locations of the majority of regular sightings.
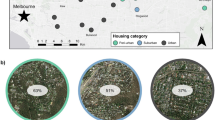
From February to May 2019, we surveyed a 60 km2 area of Greater Santo Domingo. The survey area was divided into 1 km2 grid cells using a square grid overlay on QGIS (version 2.18.16). Only cells which were (a) considered to be safe to visit, (b) possessed a suitable road grid to walk transects and (c) offered a suitable crossing if a river was present were chosen for potential surveying. Owing to the nature of the city, this restricted the survey area to the city centre, with the majority of the grid cells falling inside the Distrito Nacional (Fig. 1). We used a combination of line transects and point-counts to document Hispaniolan Parakeets in each of the grid cells (Buckland et al. 2008). Line transects were 4 km in length and passed through four of the 1 km2 squares during each survey; an additional five 1 km2 squares were surveyed using single 1 km transects. We chose this resolution based on previous research into urban parrot populations (Grarock et al. 2013, Appelt et al. 2016) and because it captured the variation between urban areas within the city while still allowing the repeated surveys to be completed in one season. Visibility may have differed between transects owing to variation in urban structure, and was likely to be limited to several hundred metres from the transect line rather than extending to the whole 1 km2. We attempted to account for these differences in detectability by using measurements of urban structure in our analyses (see below).
Number of Hispaniolan Parakeet groups encountered across three visits to each 1 km2 surveyed. Background shows urban landscape classification (water = blue, buildings and roads = grey, green spaces = green) overlaid on Open Street Map of Santo Domingo (map data copyrighted OpenStreetMap contributors and available from https://www.openstreetmap.org)
Transects were walked at a slow pace (2–3 km h−1) and repeated three times on separate days (Field et al. 2005). Five-minute point-counts, during which habitat characteristics were recorded (see next paragraph), were conducted twice in each 1 km2 square at 250 m and 500 m along the transect. During the surveys, all Hispaniolan Parakeets and Hispaniolan Woodpeckers seen and heard were recorded. Fieldworkers (LIH & CJB) were familiar with both species, which are distinctive vocally and visually: Hispaniolan Woodpecker is the only large woodpecker present on the island and Hispaniolan Parakeet is the only parakeet species present in Santo Domingo (Latta et al. 2006). Surveys were conducted during two time-slots, 7h30–9h30 and 15h00–17h00. Transect repeats meant each transect was surveyed during both time-slots at least once. These slots were selected as they avoided not only the period of lowest parakeet mobility and detectability over the hottest hours of the day but also the periods of highest mobility in the hour after sunrise and the hour before dusk, when birds are dispersing from and to their roost-sites (Marsden 1999), thus favouring detection in areas where resource use could be recorded.
Fine-scale habitat characteristics were documented during five-minute point-counts using methods adapted from MacGregor-Fors & Schondube (2011), involving a circle of 25 m radius around the point-count. The maximum building height, road width and tree species richness were recorded, with the maximum value of each characteristic per square used (from two counts per square). Tree species were identified in the field. Additionally, a Normalised Difference Vegetation Index (NDVI) value for each 1 km2 square was obtained from eMODIS (Jenkerson et al. 2010) ten-day composite NDVI V6 product for Central America and the Caribbean beginning on 26 March 2019.
Environmental data layers
To map effectively the spatial heterogeneity of urban green space cover (i.e. individual tree canopies, parks, urban woodland), high-resolution image data from the Sentinel-2 sensor (10 m; ESA 2019a) were acquired from the European Space Agency Copernicus data repository (ESA 2019b). Two image tiles from 11 January 2019, representing virtual cloud-free conditions and pre-processed to surface reflectance (ESA 2019c), were mosaiced to cover the study area. The Blue, Green, Red and Near Infrared (NIR) band layers were extracted to form a four-layer image stack. NDVI, providing a measure of active vegetation, was added as a fifth layer (NDVI = NIR – Red / NIR + Red; Braun & Herold 2004). Remaining cloud cover (≈ 0.7% of study area) was manually masked from the image stack.
To produce a map of green spaces in Santo Domingo we used a supervised classification approach (Wegmann et al., 2016). We used GoogleMaps (www.google.co.uk/maps) as a reference to develop training and testing shapefiles for three habitat classes in the city. Each file contained 20 examples of ‘Trees’, defined as either single trees or larger green spaces predominantly covered with trees, ‘Urban areas’, defined as areas covered with concrete or buildings, and ‘Water’, areas covered with water. Using the training dataset, we fitted a RandomForest (Liaw and Wiener, 2002) model which was then applied to the whole of Santo Domingo to classify habitat (Wegmann et al., 2016). Testing for agreement between habitat classifications and our ‘testing’ dataset showed excellent accuracy (0.997; 95% CI 0.992–0.999) and we also verified these classifications based on locations known to authors resident in the city and with comparisons to satellite photographs. We then used the ‘landscapemetrics’ package (Hesselbarth et al. 2019) in R to calculate the number and size of patches (discrete areas of a single land-cover classification) of ‘Trees’ within the city. We also calculated the total area of patches larger than 2 ha within the 1 km2 square to represent land covered by parks within the survey squares. Owing to the high degree of autocorrelation between landscape metrics, only the number of patches and the total area of all patches larger than 2 ha were used in our models.
Data analysis
Single-season site-occupancy models were used to investigate the distribution of parakeets and woodpeckers within Santo Domingo. Models for each species were constructed separately with the same covariates. Time of survey, distance walked, maximum building height and maximum road width were considered as potential covariates affecting detectability. Maximum tree species richness, number of discrete tree patches (>100 m2) and total area of patches larger than 2 ha within the survey square were considered as covariates affecting occupancy. Initially models containing only covariates of detectability were fitted and the ‘best’ model selected by comparing AICc scores. Then occupancy covariates were added, keeping detectability covariates constant, and selected in the same manner. In both cases, models containing all combinations of covariates were fitted and compared. Models with ΔAICc <2 were considered to have equal support. In this case the simplest models were considered to be ‘best’ (Burnham & Anderson 1998).
Relative abundance of parakeet encounters (i.e. number of groups encountered in each survey square) was modelled using N-Mixture models (Royle 2004). It was not possible to model total abundance of birds, because detectability could not be assumed to be equal for each bird in any given flock (Kéry & Royle 2015). We used the same potential covariates for detectability as in the site-occupancy models, and the covariates which could potentially influence occupancy were also used to investigate their potential relationship with relative abundance. The same model selection method, first involving covariates for detectability followed by covariates for relative abundance, was also used. All models were fitted in R (version 4.0; R Core Team 2020) using the unmarked package (Fiske & Chandler 2011). All of our ‘best’ models were checked with goodness-of-fit tests (MacKenzie & Bailey 2004) using the AICcMODAvg package (Mazerolle 2019).
Results
Parakeet distribution and abundance
Parakeets were observed in 32 out of 60 1 km2 survey squares (Fig. 1). There was no difference in the distribution of parakeet presence across the city (Chi-squared test between number of ‘occupied’ squares across the NE, NW, SE and SW quadrants: χ23 = 3.5, p = 0.32). However, parakeet records were concentrated in the south of the city in areas surrounding a linear urban park called Parque del Mirador Sur (χ23 = 82, p < 0.001). The ‘best’ model (Tables 1 & 2 SOM) for parakeet occupancy (Fig. 2a) used no covariates influencing detectability (0.56 ± 0.06), and a positive relationship with maximum tree species richness (0.27 ± 0.13; Fig. 2b) as a predictor of occupancy (0.82 ± 0.11).
Results of analysis of the distribution of Hispaniolan Parakeet in Santo Domingo, Dominican Republic, showing (a) Predicted parakeet occupancy (darker blue indicates a higher probability of occupancy), (b) Probability of occupancy based on the maximum tree species richness, (c) Predicted number of parakeet encounters (darker blue indicates higher encounter rates) and (d) Predicted detectability based on maximum road width and number of encounters based on maximum tree species richness and number of tree patches within the survey square. On maps, letters represent A the colonial roost at El Embajador Hotel, B the nesting site at UASD and C the nesting site at Hospital San Nicholás de Bari
The majority of parakeet records were of single individuals, but 46 groups were recorded in total, with a median group size of three. Mean group size across the three repeated surveys was correlated with parakeet encounter rates (rs = 0.95, n = 60, p < 0.001) and the total encounters were positively correlated between visits in each location (1&2 rs = 0.59, n = 60, p < 0.001; 1&3 rs = 0.56, n = 60, p < 0.001; 2&3 rs = 0.47, n = 60, p < 0.001). The ‘best’ model explaining parakeet encounter rates (Fig. 2c; Tables 3 & 4 SOM) used a negative binomial error structure due to overdispersion (Kéry & Royle 2015). Maximum road width (−0.014 ± 0.01; Fig. 2d) was selected as a covariate for detectability (0.06 ± 0.02) and maximum tree species richness (0.19 ± 0.079; Fig. 2d) and number of tree patches within the survey area (0.017 ± 0.007; Fig. 2d) were selected as covariates influencing the number of parakeet encounters. There was only moderate correlation between predicted probability of occupancy and predicted encounter rates (rs = 0.49).
Nesting and roosting ecology
Breeding was recorded at two well-known sites, the Hospital San Nicolás de Bari ruins in the Ciudad Colonial and the Pedro Mir Library, Universidad Autónoma de Santo Domingo (UASD) (Fig. 2). Breeding was also found close to the Jardín Botánico Nacional, another known site, in the form of a single nest in a cavity in a mature palm. No further nests were located during the transects or by questioning local people. The cavities are attended by parakeets in every month of the year, although breeding is believed to take place mainly from February to June (Latta et al. 2006).
Hispaniolan Woodpecker was recorded in many areas across the city, as were woodpecker cavities which could provide potential nest-sites for parakeets. In some areas, such as within the Jardín Botánico Nacional, multiple cavities were present in the same tree. However, we saw no evidence that use of these cavities by parakeets was common. The ‘best’ model (Table 5 & 6; Online resource 1) demonstrated that woodpecker occupancy (0.44 ± 0.15; Fig. i; Online resource 1) was positively related to maximum tree species richness (0.37 ± 0.19; Fig. iia; Online resource 1) and, unlike the parakeet encounters model, negatively related to the number of tree patches within the survey square (−0.04 ± 0.02; Fig. iib; Online resource 1) as predictors of occupancy (0.44 ± 0.15).
There is just one known roost site regularly used by parakeets in Santo Domingo. This is located at the entrance to El Embajador Hotel at the east end of the Parque del Mirador Sur. Here, parakeets congregate for the night in four mature Indian almond Terminalia catappa trees. Our own counts and those from previous visits (Table 7; Online resource 1) suggest that more than 1500 individuals can regularly use this roost, along with very small numbers of Hispaniolan Amazons. The urban population is likely to be larger than this, since birds were observed at the two known breeding colonies at times when the roost at El Embajador Hotel was assembling, and other parrot species are known to be absent from communal roosts when breeding (Cougill & Marsden 2004).
Discussion
The Hispaniolan Parakeet proved to be widespread within our 60 km2 study area in central Santo Domingo. The species shows a rather patchy distribution across the rest of Hispaniola, with the highest proportion of eBird lists placing it in the provinces of Independencia and Pedernales in south-west DR plus the Département du Nord in Haiti. Even so, in these areas it is recorded on only around 15% of lists, whereas in Santo Domingo it is recorded on over 50% of lists; and given a detectability in our models of 0.56, it is probably ‘present’ in most locations. There is little evidence from eBird records, at least, that urban areas on other Caribbean islands support parakeet populations in any way similar to the situation in Santo Domingo. Psittacara species are present in urban areas in other parts of Latin America (e.g. Finsch’s Parakeet P. finschi and White-eyed Parakeet P. leucophthalmus), but these urban populations appear to represent part of continuous ranges rather than a disjunct ‘self-contained’ population such as is present in Santo Domingo.
Intriguingly, the city proves to have been colonised only recently by the parakeet. It was not mentioned as a locality in their meticulous account of Hispaniolan birds by Wetmore & Swales (1931) or Wiley (1991). Moreover, the roost at El Embajador Hotel consisted of just 118 birds in 1998, when it was described as ‘the largest flock recorded in recent years, and particularly unusual in being in an urban area’ (Kirwan et al. 1999). As our surveys indicate a far larger population of 1500+ birds within the city, again centred in the south, with that number regularly roosting at El Embajador Hotel, it can plausibly be construed that the population became established in the mid-1990s (presumably from released or escaped market birds: see below) and grew very rapidly, with some occupants of city apartments now even feeding the birds on their balconies (SG pers. info.). Latta et al. (2006) referred to the species as ‘common in urban parks in Santo Domingo’ and, although we did not find a particular association with large parks, we can confirm that green spaces throughout the city are important drivers of their urban distribution.
Specifically, records were centred on those areas with high richness of tree species, and encounter rates with parakeet groups were positively related to tree species richness along with number of green spaces within the area. Despite this, and although the size of urban green spaces generally influences bird species richness (Dale 2018), we found no strong link between either parakeet distribution or encounter rates and proximity to the city’s largest green spaces. Within urban areas, tree species richness can be greater than surrounding native forest, but the proportion of non-native species also increases (Nero et al. 2017). Urban parrot species are reported to use a variety of both native and non-native tree species (Groom et al. 2014), including those found in ornamental green spaces such as parks and gardens. Opportunistic observations of parakeets feeding on a number of exotic trees in Parque del Mirador Sur during the past three years confirm this (YML, pers. obs.). The combination of native and non-native vegetation may increase both the variety and seasonal abundance of available food resources (Lowry and Lill 2007), permitting higher population densities of psittacids than in rural areas (Sol et al. 2013). This could explain why the Jardín Botánico Nacional, with its high woodland cover and many native tree species, had relatively low parakeet encounter rates, possibly because it has fewer non-native trees planted for their fruits, such as mangoes (much exploited by the parakeet, as observed on our surveys), than small urban parks, public gardens and private courtyards. In our view, parakeets occupy the central areas of Santo Domingo simply because smaller, diverse green spaces are more plentiful there than in peripheral suburbs.
These central areas are also close to the single known large communal roost, which may play an important role in the success of the city’s population. Roosts such as this allow the transmission of key information, notably the location of foraging areas (Bijleveld et al. 2010). High inter-individual variability in behaviours in parrot species may also allow the rapid development of new adaptations in urban environments (Carrete and Tella, 2011), in our case such as breeding in buildings, and the well-developed cognitive abilities of parrots (Schuck-Paim et al. 2008) would allow these novel behaviours to be quickly transmitted through the population. Indeed, gregariousness is associated with the ability to find and exploit novel foods (Chapman et al. 1989, Sol et al. 2013), such as those from unfamiliar tree species. The roost at El Embajador Hotel offers opportunities to monitor this important urban population (e.g. Pithon & Dytham 1999).
The distribution of Hispaniolan Woodpeckers was similarly driven by tree species richness, but was negatively related to the number of tree patches in the survey square, suggesting a need for less fragmented areas. Nevertheless, despite the widespread availability of old woodpecker holes, we found only one such being used by parakeets. The distributions of other urban parakeet species are believed to be constrained by the availability of nesting cavities (Strubbe & Matthysen 2009, Davis et al. 2012), but for Hispaniolan Parakeets there appears to be an abundance of ostensibly suitable but unused nest-sites. Based on roost counts of over 1500 individuals, it would appear that, unless there are several major undetected breeding colonies, only a small proportion of the population breeds at any one time. After the fieldwork was completed, two additional nests were detected in building structures in the Distrito Nacional: one in a cement shelf for air-conditioning units outside an apartment building and another on a hollow ceiling structure outside a shopping centre (YML pers. obs.). Such nests, however, seem to be rare. If this is so, the two known communal nesting sites, both in buildings, are of vital importance to the species.
The origin of the parakeet population in Santo Domingo (and other urban areas in the DR) remains an open question. However, the evidence is consistent with the population deriving from escaped or released birds. A long tradition exists among Dominicanos of keeping the endemic Hispaniolan Amazon, which can be taught to ‘talk’ well, as a pet; parakeets, by contrast, do not learn to talk but have unpleasantly loud and persistent calls. Nevertheless, fledgling parakeets, which are visually similar to fledgling amazons, are often sold along the city’s approach roads to unsuspecting purchasers (YML pers. obs.), and it is entirely plausible that disappointed or exasperated owners released such birds when they discovered their mistake. Moreover, in the mid-1990s the city zoo received so many donations and confiscations of parakeets that staff reputedly contemplated their release as a humanitarian option (SG pers. info., S. J. Inchaustegui in litt.). Given the rarity of the species outside of the city now, the source of these broods is a matter of considerable interest.
Regardless of their origin, Santo Domingo represents a stronghold for the Hispaniolan Parakeet, at least currently. In other areas, threatened species may be less dependent on the urban parts of their range but the situation in Santo Domingo demonstrates the potential importance of such populations, and their relevance is only likely to increase if trends related to urban living and expansion continue. It is certainly important to study the population ecology of the Hispaniolan Parakeet in Santo Domingo in order to develop evidence-based long-term conservation management initiatives. Whether this urban population can provide information to help protect populations in the wider countryside is still unclear. Loss of parakeet populations may have resulted in the loss of ecosystem function from their original range (Luna et al. 2018). We do not know if the city currently provides all of the necessary resources for this parakeet population to thrive or whether the availability of some, but not all, required resources represents an ecological trap (Battin 2004). While we have highlighted areas of the city and resources which may be important to this population, exactly how and when birds are using those resources needs study. The tagging of birds at colonies and roost(s) would offer a way to track movements around the urban environment, identify key foraging resources and generate vital demographic data. Nest monitoring and, potentially, the provision of protected, artificial nest sites could help determine the natural and anthropogenic pressures governing reproductive behaviour and output, and could be deployed in other urban areas within the DR. Work of this nature might also shed light on the causes of the poor status of the species in the Hispaniolan countryside, and might be used as the scientific backdrop to the greater engagement of the public in support of such a familiar, yet virtually unknown, islandwide endemic species.
References
Alberti M (2005) The effects of urban patterns on ecosystem function. Int Reg Sci Rev 28:168–192
Alvey AA (2006) Promoting and preserving biodiversity in the urban forest. Urban For Urban Green 5:195–201
Appelt CW, Ward LC, Bender C, Fasenella J, Vossen BJV, Knight L (2016) Examining potential relationships between exotic monk parakeets (Myiopsitta monachus) and avian communities in an urban environment. Wilson J Ornithol 128(3):556–566
Battin J (2004) When good animals love bad habitats: ecological traps and the conservation of animal populations. Conserv Biol 18(6):1482–1491
BirdLife International (2020) Species factsheet: Psittacara chloropterus. Downloaded from http://www.birdlife.org on 1 June 2020
Bijleveld AI, Egas M, Van Gils JA, Piersma T (2010) Beyond the information centre hypothesis: communal roosting for information on food, predators, travel companions and mates? Oikos 119(2):277–285
Braun M, Herold M (2004) Mapping imperviousness using NDVI and linear spectral unmixing of ASTER data in the Cologne-Bonn region (Germany). In Remote Sensing for Environmental Monitoring, GIS Applications, and Geology III. International Society for Optics and Photonics 5239:274–284
Buckland ST, Marsden SJ, Green RE (2008) Estimating bird abundance: making methods work. Bird Conserv Int 18(1):91–108. https://doi.org/10.1017/S0959270908000294
Butler CJ (2005) Feral parrots in the continental United States and United Kingdom: past, present, and future. J Avian Med Surg 19:142–149
Burnham KP, Anderson DR (1998) Model selection and inference: a practical information-theoretical approach. Springer-Verlag, New York
Carrete M, Tella JL (2011) Inter-individual variability in fear of humans and relative brain size of the species are related to contemporary urban invasion in birds. PLoS One 6(4):e18859
Chapman CA, Chapman LJ, Lefebvre L (1989) Variability in parrot flock size: possible functions of communal roosts. Condor 91(4):842–847
Clergeau P, Vergnes A (2011) Bird feeders may sustain feral rose-ringed parakeets Psittacula krameri in temperate Europe. Wildl Biol 17:248–252
Collar NJ, Crosby MJ, Stattersfield AJ (1994) Birds to watch 2: the world list of threatened birds. BirdLife International (BirdLife Conservation Series 4), Cambridge, U.K.
Collar NJ, Boesman PFD, Sharpe CJ, Greeney HF (2020) Hispaniolan parakeet (Psittacara chloropterus), version 1.0. In: Billerman SM, Keeney BK, Rodewald PG, Schulenberg TS (eds) Birds of the World. Cornell Lab of Ornithology, Ithaca, NY, USA. https://doi.org/10.2173/bow.hispar.01
Cougill S, Marsden SJ (2004) Variability in roost size in an Amazona parrot: implications for roost monitoring. J Field Ornithol 75(1):67–73
Dale S (2018) Urban bird community composition influenced by size of urban green spaces, presence of native forest, and urbanization. Urban Ecosyst 21(1):1–14
Davis A, Taylor CE, Major RE (2012) Seasonal abundance and habitat use of Australian parrots in an urbanised landscape. Landsc Urban Plan 106:191–198
DeStefano S, DeGraaf RM (2003) Exploring the ecology of suburban wildlife. Front Ecol Environ 1:95–101
Dod AS (1992) Endangered and endemic birds of the Dominican Republic. Cypress House, Fort Bragg, California
ESA [European Space Agency] (2019a) Sentinel-2 MSI: Overview. Online resource retrieved from: https://earth.esa.int/web/sentinel/user-guides/sentinel-2-msi/overview. Accessed 23 Dec 2019
ESA [European Space Agency] (2019b) Copernicus Open Access Hub. Online resource retrieved from: https://scihub.copernicus.eu/. Accessed 23 Dec 2019
ESA [European Space Agency] (2019c) Sentinel online: User Guides: Level-2. Online resource retrieved from: https://sentinel.esa.int/web/sentinel/user-guides/sentinel-2-msi/processing-levels/level-2. Accessed 23 Dec 2019
Field S, Tyre A, Possingham H (2005) Optimizing allocation of monitoring effort under economic and observational constraints. J Wildl Manag 69(2):473–482. https://doi.org/10.2193/0022-541x(2005)069[0473:oaomeu]2.0.co;2
Fiske I, Chandler R (2011) unmarked: An R Package for Fitting Hierarchical Models of Wildlife Occurrence and Abundance. J Stat Softw 43(10):1–23. URL http://www.jstatsoft.org/v43/i10/
Grarock K, Lindenmayer DB, Wood JT, Tidemann CR (2013) Does human-induced habitat modification influence the impact of introduced species? A case study on cavity-nesting by the introduced common myna (Acridotheres tristis) and two Australian native parrots. Environ Manag 52(4):958–970
Groom CJ, Mawson PR, Roberts JD, Mitchell NJ (2014) Meeting an expanding human population’s needs whilst conserving a threatened parrot species in an urban environment. WIT Trans Ecol Environ 191:1199–1212
del Hoyo J, Collar NJ (2014) The HBW–BirdLife International illustrated checklist of the birds of the world, 1: non-passerines. Lynx Edicions, Barcelona
Hesselbarth MHK, Sciaini M, With KA, Wiegand K, Nowosad J (2019) Landscapemetrics: an open-source R tool to calculate landscape metrics. Ecography 42:1648–1657
Irumba IO, Pomeroy D, Perrin M (2016) Grey parrots Psittacus erithacus in Kampala, Uganda—are they becoming suburbanised? Ostrich 87:193–195
Ives CD, Lentini PE, Threlfall CG, Ikin K, Shanahan DF, Garrard GE, Bekessy SA, Fuller RA, Mumaw L, Rayner L, Rowe R (2016) Cities are hotspots for threatened species. Glob Ecol Biogeogr 25(1):117–126
Jenkerson CB, Maiersperger TK, Schmidt GL (2010) eMODIS: a user-friendly data source; open-file report 2010–1055. US Geological Survey: Reston, VA, USA
Keith AR, Wiley JW, Latta SC, Ottenwalder JA (2003) The birds of Hispaniola: an annotated checklist. British Ornithologists’ Union (checklist 21), Tring
Kéry M, Royle JA (2015) Applied hierarchical modeling in ecology: analysis of distribution, abundance and species richness in R and BUGS: volume 1: prelude and static models. Academic Press
Kirwan GM, Williams RSR, Bradshaw CG (1999) Interesting avifaunal records from the Dominican Republic. Cotinga 11:27–29
Kirwan GM, Levesque A, Oberle M, Sharpe CJ (2019) The birds of the West Indies. Lynx Edicions, Barcelona
Latta S, Rimmer C, Keith A, Wiley J, Raffaele H, McFarland K, Fernandez E (2006) Birds of the Dominican Republic and Haiti. Christopher Helm, London
Liaw A, Wiener M (2002) Classification and regression by randomForest. R News 2(3):18–22
Lowry H, Lill A (2008) Ecological factors facilitating city-dwelling in red-rumped parrots. Wildl Res 34(8):624–631
Luna A, Romero-Vidal P, Hiraldo F, Tella JL (2018) Cities may save some threatened species but not their ecological functions. Peer J 6:e4908
MacGregor-Fors I, Schondube J (2011) Gray vs. green urbanization: relative importance of urban features for urban bird communities. Basic Appl Ecol 12(4):372–381. https://doi.org/10.1016/j.baae.2011.04.003
MacKenzie DI, Bailey LL (2004) Assessing the fit of site-occupancy models. J Agric Biol Environ Stat 9(3):300–318
Marsden SJ (1999) Estimation of parrot and hornbill densities using a point-count distance sampling method. Ibis 141(3):377–390. https://doi.org/10.1111/j.1474-919x.1999.tb04405.x
Martens JM, Woog F (2017) Nest cavity characteristics, reproductive output and population trend of naturalised amazon parrots in Germany. J Ornithol 158(3):823–832. https://doi.org/10.1007/s10336-017-1436-9
Mazerolle MJ (2019) AICcmodavg: model selection and multimodel inference based on (Q)AIC(c). R package version 2.2–2. https://cran.r-project.org/package=AICcmodavg
Nero BF, Campion BB, Agbo N, Callo-Concha D, Denich M (2017) Tree and trait diversity, species coexistence, and diversity-functional relations of green spaces in Kumasi, Ghana. Proced Eng 198:99–115
Olson SL (2015) History, morphology, and fossil record of the extinct Puerto Rican parakeet Psittacara maugei Souancé. Wilson J Ornithol 127(1):1–12
Pithon JA, Dytham C (1999) Census of the British ring-necked parakeet Psittacula krameri population by simultaneous counts of roosts. Bird Stud 46:112–115
Population Stat (2019) Santo Domingo, Dominican Republic Population. Retrieved from: https://populationstat.com/dominican-republic/santo-domingo
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for statistical computing, Vienna, Austria. URL https://www.R-project.org/
Royle JA (2004) N-mixture models for estimating population size from spatially replicated counts. Biometrics 60(1):108–115
Salinas-Melgoza A, Salinas-Melgoza V, Wright TF (2013) Behavioral plasticity of a threatened parrot in human-modified landscapes. Biol Conserv 159:303–312
Schuck-Paim C, Alonso WJ, Ottoni EB (2008) Cognition in an ever-changing world: climatic variability is associated with brain size in neotropical parrots. Brain Behav Evol 71(3):200–215
Sol D, Lapiedra O, González-Lagos C (2013) Behavioural adjustments for a life in the city. Anim Behav 85(5):1101–1112
Snyder N, McGowan P, Gilardi J, Grajal A (2000) Parrots: status survey and conservation action plan 2000–2004. IUCN, Gland
Stattersfield AJ, Capper DR (eds) (2000) Threatened birds of the world. BirdLife International, Cambridge, U.K., and Lynx Edicions, Barcelona
Stofberg M, Cunningham SJ, Sumasgutner P, Amar A (2019) Juggling a ‘junk-food’ diet: responses of an urban bird to fluctuating anthropogenic-food availability. Urban Ecosyst 22(6):1019–1026. https://doi.org/10.1007/s11252-019-00885-3
Strubbe D, Matthysen E (2009) Predicting the potential distribution of invasive ring-necked parakeets Psittacula krameri in northern Belgium using an ecological niche modelling approach. Biol Invasions 11(3):497–513
Tella JL, Canale A, Carrete M, Petracci P, Zalba SM (2014) Anthropogenic nesting sites allow urban breeding in burrowing parrots Cyanoliseus patagonus. Ardeola 61:311–321
Wegmann M, Leutner B, Dech S (eds) (2016) Remote sensing and GIS for ecologists: using open source software. Pelagic Publishing Ltd
Wetmore A, Swales, BH (1931) The birds of Haiti and the Dominican Republic. Bull. U.S. Natn. Mus. 155
Wiley JW (1991) Status and conservation of parrots and parakeets in the greater Antilles, Bahama Islands, and Cayman Islands. Bird Conserv Int 1:187–214
Acknowledgments
We express our thanks to Chester University for funding, and to Eric Vignot (Parrot Wildlife Foundation) for his support of our work in the Dominican Republic; to Grupo Jaragua for logistical support; to Eladio Fernández, Sixto Inchaustegui, Allan Keith, Guy Kirwan, Steve Latta, Helen Temple and local birders and students for assistance with information and fieldwork; and to two anonymous reviewers who helped improve the manuscript.
Availability of data and material
Data are available on request.
Code availability
Code is available on request.
Funding
This research was supported by the Department of Biological Sciences, University of Chester. Our work in the Dominican Republic is also supported by the Parrot Wildlife Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest/competing interests
None of the authors has any conflicting or competing interests.
Ethics approval
This research was approved by the Research Ethics Committee for the Faculty of Medicine, Dentistry and Life Sciences at the University of Chester.
Supplementary Information
ESM 1
(PDF 915 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Geary, M., Brailsford, C.J., Hough, L.I. et al. Street-level green spaces support a key urban population of the threatened Hispaniolan parakeet Psittacara chloropterus. Urban Ecosyst 24, 1371–1378 (2021). https://doi.org/10.1007/s11252-021-01119-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-021-01119-1