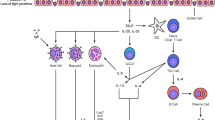
Abstract
Background
Asthma is one of the most common noninfectious chronic diseases characterized by type II inflammation. This study aimed to investigate the effects of molecular hydrogen on the pathogenesis of asthma.
Methods
OVA sensitized asthma mouse model and house dust mite treated 16HBE cellular model were established and hydrogen/oxygen mixture was used to treat asthmatic mice and 16HBE cells. Serum and BALF cytokines were measured with specific ELISA assays. E-cadherin and ZO-1 were detected by immunohistochemical staining and expression of caspase 3 and 9, NF-κB, IL-33 and ST2 was assessed by quantitative real-time PCR, western blot and/or immunofluorescence. IL-33 promoter activity was analyzed by dual-luciferase assay. ILC2 population was assayed by flow cytometry and differentially expressed miRNAs were detected using miRNA array.
Results
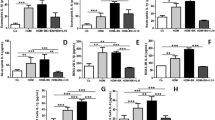
Serum and BALF levels of IL-33 and other alarmin and type II cytokines were greatly increased by OVA and inhibited by H2 in asthmatic mice. The expression of NF-κB (p65) and ST2 was upregulated by OVA and suppressed by H2. ILC2 population was markedly increased in OVA-induced asthmatic mice, and such increase was inhibited by H2. E-cadherin and ZO-1 levels in airway tissues of asthmatic mice were significantly lower than that of control mice, and the reduction was recovered by H2 treatment. H2 alleviated HDM induced apoptosis of 16HBE cells, upregulation of IL-33 and ST2, and elevation of IL-33 promoter activity. A group of miRNAs differentially expressed in HDM and HDM + H2 treated 16HBE cells were identified.
Conclusions
These data demonstrated that H2 is efficient in suppressing allergen-induced asthma and could be developed as a therapeutics for asthma and other conditions of type II inflammation.







Similar content being viewed by others
References
Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–96.
Khalaf K, Paoletti G, Puggioni F, Racca F, De Luca F, Giorgis V, et al. Asthma from immune pathogenesis to precision medicine. Semin Immunol. 2019;46:101294.
Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. 2018;391:783–800. https://doi.org/10.1016/S0140-6736(17)33311-1.
Kubo M. Innate and adaptive type 2 immunity in lung allergic inflammation. Immunol Rev. 2017;278:162–72.
Kim HY, DeKruyff RH, Umetsu DT. The many paths to asthma: phenotype shaped by innate and adaptive immunity. Nat Immunol. 2010;11:577–84.
Pulendran B, Artis D. New paradigms in type 2 immunity. Science. 2012;337:431–5.
Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, et al. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol. 2002;169:443–53.
Halim TYF, McKenzie ANJ. New kids on the block: group 2 innate lymphoid cells and type 2 inflammation in the lung. Chest. 2013;144:1681–6.
Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–70.
Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107:11489–94.
Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105–16.
Herbert DR, Douglas B, Zullo K. Group 2 Innate Lymphoid Cells (ILC2): Type 2 immunity and Helminth immunity. Int J Mol Sci. 2019;20:2276.
Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–63.
Mjösberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–62.
Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci U S A. 2009;106:9021–6.
Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–94.
Ge L, Yang M, Yang NN, Yin XX, Song WG. Molecular hydrogen: a preventive and therapeutic medical gas for various diseases. Oncotarget. 2017;8:102653–73.
LeBaron TW, Kura B, Kalocayova B, Tribulova N, Slezak J. A new approach for the prevention and treatment of cardiovascular disorders. Molecular hydrogen significantly reduces the effects of oxidative stress. Molecules. 2019;24:2076.
Kura B, Bagchi AK, Singal PK, Barancik M, LeBaron TW, Valachova K, et al. Molecular hydrogen: potential in mitigating oxidative-stress-induced radiation injury 1. Can J Physiol Pharmacol. 2019;97:287–92.
Cejka C, Kossl J, Hermankova B, Holan V, Kubinova S, Zhang JH, et al. Therapeutic effect of molecular hydrogen in corneal UVB-induced oxidative stress and corneal photodamage. Sci Rep. 2017;7:18017.
Aldakheel FM, Thomas PS, Bourke JE, Matheson MC, Dharmage SC, Lowe AJ. Relationships between adult asthma and oxidative stress markers and pH in exhaled breath condensate: a systematic review. Allergy. 2016;71:741–57.
Sahiner UM, Birben E, Erzurum S, Sackesen C, Kalayci Ö. Oxidative stress in asthma: part of the puzzle. Pediatr Allergy Immunol. 2018;29:789–800.
Fekonja S, Korošec P, Rijavec M, Jeseničnik T, Kunej T. Asthma microRNA regulome development using validated miRNA-target interaction visualization. OMICS. 2018;22:607–15.
Maes T, Cobos FA, Schleich F, Sorbello V, Henket M, De Preter K, et al. Asthma inflammatory phenotypes show differential microRNA expression in sputum. J Allergy Clin Immunol. 2016;137:1433–46.
Kato A. Group 2 innate lymphoid cells in airway diseases. Chest. 2019;156(1):141–9.
Boonpiyathad T, Sözener ZC, Satitsuksanoa P, Akdis CA. Immunologic mechanisms in asthma. Semin Immunol. 2019;46:101333.
Magat JM, Thomas JL, Dumouchel JP, Murray F, Li WX, Li J. Endogenous IL-33 and its autoamplification of IL-33/ST2 pathway play an important role in asthma. J Immunol. 2020;204:1592–7.
Specjalski K, Jassem E. MicroRNAs: potential biomarkers and targets of therapy in allergic diseases? Arch Immunol Ther Exp (Warsz). 2019;67:213–23.
Mousavi SR, Ahmadi A, Jamalkandi SA, Salimian J. Involvement of microRNAs in physiological and pathological processes in asthma. J Cell Physiol. 2019;234:21547–59.
Liu F, Qin HB, Xu B, Zhou H, Zhao DY. Profiling of miRNAs in pediatric asthma: upregulation of miRNA-221 and miRNA-485-3p. Mol Med Rep. 2012;6:1178–82.
Panganiban RP, Wang Y, Howrylak J, Chinchilli VM, Craig TJ, August A, et al. Circulating microRNAs as biomarkers in patients with allergic rhinitis and asthma. J Allergy Clin Immunol. 2016;137:1423–32.
Lacedonia D, Palladino GP, Foschino-Barbaro MP, Scioscia G, Carpagnano GE. Expression profiling of miRNA-145 and miRNA-338 in serum and sputum of patients with COPD, asthma, and asthma-COPD overlap syndrome phenotype. Int J Chron Obstruct Pulmon Dis. 2017;12:1811–7.
Suojalehto H, Lindström I, Majuri ML, Mitts C, Karjalainen J, Wolff H, et al. Altered microRNA expression of nasal mucosa in long-term asthma and allergic rhinitis. Int Arch Allergy Immunol. 2014;163:168–78.
Solberg OD, Ostrin EJ, Love MI, Peng JC, Bhakta NR, Hou L, et al. Airway epithelial miRNA expression is altered in asthma. Am J Respir Crit Care Med. 2012;186:965–74.
Kim RY, Horvat JC, Pinkerton JW, Starkey MR, Essilfie AT, Mayall JR, et al. MicroRNA-21 drives severe, steroid-insensitive experimental asthma by amplifying phosphoinositide 3-kinase-mediated suppression of histone deacetylase 2. J Allergy Clin Immunol. 2017;139:519–32.
Roan F, Obata-Ninomiya K, Ziegler SF. Epithelial cell-derived cytokines: more than just signaling the alarm. J Clin Invest. 2019;129:1441–51.
Sugita K, Steer CA, Martinez-Gonzalez I, Altunbulakli C, Morita H, Castro-Giner F, et al. Type 2 innate lymphoid cells disrupt bronchial epithelial barrier integrity by targeting tight junctions through IL-13 in asthmatic patients. J Allergy Clin Immunol. 2018;141:300–10.
Yuan X, Wang J, Li Y, He X, Niu B, Wu D, et al. Allergy immunotherapy restores airway epithelial barrier dysfunction through suppressing IL-25 -induced endoplasmic reticulum stress in asthma. Sci Rep. 2018;8:7950.
Gon Y, Hashimoto S. Role of airway epithelial barrier dysfunction in pathogenesis of asthma. Allergol Int. 2018;67:12–7.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, J., Feng, X., Fan, Y. et al. Molecular hydrogen alleviates asthma through inhibiting IL-33/ILC2 axis. Inflamm. Res. 70, 569–579 (2021). https://doi.org/10.1007/s00011-021-01459-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-021-01459-w