Abstract
Orthostatic hypotension (OH) is relatively common in the early stage of Parkinson’s disease (PD). It is divided into delayed OH and classical OH. Classical OH in PD has been investigated widely, however, the clinical implications of delayed OH in PD have seldom been studied. The purpose of this study is to characterize delayed OH in PD. A total of 285 patients with early drug-naïve PD were enrolled and divided into three groups according to orthostatic change: no-OH, delayed OH, and classical OH. The disease severity in terms of motor, non-motor, and cognitive functions was assessed. The cortical thickness of 82 patients was analyzed with brain magnetic resonance imaging. The differences among groups and linear tendency in the order of no-OH, delayed OH, and classical OH were investigated. Seventy-seven patients were re-evaluated. Initial and follow-up evaluations were explored to discern any temporal effects of orthostasis on disease severity. Sixty-four (22.5%) patients were defined as having delayed OH and 117 (41.1%) had classical OH. Between-group comparisons revealed that classical OH had the worst outcomes in motor, non-motor, cognitive, and cortical thickness, compared to the other groups. No-OH and delayed OH did not differ significantly. Linear trends across the pre-ordered OH subtypes found that clinical parameters worsened along with the orthostatic challenge. Clinical scales deteriorated and the linear gradient was maintained during the follow-up period. This study suggests that delayed OH is a mild form of classical OH in PD. PD with delayed OH has milder disease severity and progression.
Similar content being viewed by others
Introduction
Dysautonomia is a well-known non-motor feature that is discovered in the prodrome of Parkinson’s disease (PD)1,2. Orthostatic hypotension (OH) is found in early drug-naïve PD3, and its presence is consistently associated with worse outcomes4,5,6,7.
OH is clinically divided into classical OH and delayed OH8,9. Classical OH is conventionally defined as sustained decrease in systolic blood pressure (SBP) ≥ 20 mmHg and/or diastolic blood pressure (DBP) 10 mmHg within 3 min of standing, and delayed OH is considered when the progressive blood pressure drop surpasses the margin of change after 3 min. The clinical implications of delayed OH are suggested to be a non-benign, mild, or early form of sympathetic adrenergic dysfunction10. Although its significance was seldom studied in populations with PD, a longitudinal follow-up study revealed some individuals with delayed OH progressed to classical OH and developed α-synucleinopathy11.
The assumption of present study was that delayed OH is a mild form of neurogenic OH in early PD, representing milder disease severity. Patients were sub-grouped into a hypothesis-driven ordinal scale. The expected outcomes were between-group differences and a linear gradient across the subtypes, and results that were maintained with time. This would prove that patients with delayed OH have different motor and non-motor phenotypes and a distinct prognosis from other groups.
Results
Baseline characteristics
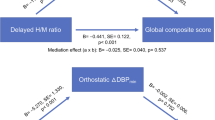
The flow of participants is presented in Fig. 1 and the baseline demographics of PD patients are summarized in Table 1. The mean age was 69.6 ± 9.3 years old and 132 (46.3%) were female. The median duration of disease was 1 (1.0) year (interquartile range, IQR). The mean total sum of Unified Parkinson’s Disease Rating Scale (UPDRS) was 23.3 ± 12.6 with a median modified Hoehn and Yahr (H&Y) of 2.0 (IQR, 1.0). The mean score of Mini-Mental Status Examination (MMSE) was 27.0 ± 2.8 and the median Clinical Dementia Rating (CDR) was 0.5 (IQR, 0). Sixty-four patients (22.5%) were defined to have delayed OH and 117 (41.1%) had classical OH. The mean uptake of delayed heart-to-mediastinum (H/M) ratio was 1.55 ± 0.37. Classical OH were older than other groups.
NMSS Non-Motor Symptoms Scale, PDQ-39 Parkinson’s Disease Quality of Life-39, OHQ Orthostatic Hypotension Questionnaire, MADRS Montgomery-Asberg Depression Rating Scale, RBDSQ REM Sleep Behavior Disorder Screening Questionnaire, UPDRS Unified Parkinson’s Disease Rating Scale, MMSE Mini-Mental Status Examination.
Comparisons across orthostatic hypotension groups
Between-group comparisons, adjusted for age, sex, and disease duration were analyzed and encapsulated in Table 2 and Supplementary Table 1. Classical OH scored higher in UPDRS Part II (no-OH vs. delayed OH vs. classical OH: 5.2 ± 0.4 vs. 5.1 ± 0.5 vs. 6.8 ± 0.4; p = 0.005; a = b < c), Part III (no-OH vs. delayed OH vs. classical OH: 14.9 ± 0.8 vs. 13.9 ± 1.1 vs 17.9 ± 0.8; p = 0.008; a = b < c), and UPDRS total score (no-OH vs. delayed OH vs. classical OH: 21.4 ± 1.1 vs. 20.7 ± 1.5 vs. 26.4 ± 1.1; p = 0.002; a < c). The classical OH group also had higher supine systolic blood pressure than the no-OH group. Of 285 PD, 154 (54.0%) had nocturnal hypertension and 138 (48.4%) were non-dippers. UPDRS scores, supine blood pressure (BP), the proportions of nocturnal hypertension, and non-dipper all showed an ascending linear trend across the OH groups.
Classical OH had higher Non-Motor Symptoms Scale (NMSS) scores (no-OH vs. classical OH: 30.2 ± 4.5 vs. 47.1 ± 4.6; p = 0.032), OHQ Part II (no-OH vs. classical OH: 4.7 ± 1.1 vs. 8.7 ± 1.1; p = 0.026), and Rapid-eye-movement Sleep Behavior Disorder Screening Questionnaire (RBDSQ; no-OH vs. classical OH: 2.5 ± 0.3 vs. 3.8 ± 0.3; p = 0.025) than no-OH. A positive linear trend was found across the groups in the NMSS, Orthostatic Hypotension Questionnaire (OHQ) Part II, Montgomery-Asberg Depression Rating Scale (MADRS), and RBDSQ questionnaires.
Cortical thickness comparison also depicted a similar pattern of between-group differences, when controlled for age, sex, disease duration, and MMSE. The whole cerebral cortex (no-OH vs. classical OH: 3.27 ± 0.02 vs. 3.15 ± 0.03; p = 0.002), in particular the frontal and parietal lobes (no-OH vs. classical OH: 3.17 ± 0.02 vs. 3.05 ± 0.02, p = 0.001; 2.98 ± 0.03 vs. 2.79 ± 0.05, p = 0.007; respectively), manifested thinner thickness in patients with classical OH group compared to no-OH. There was a negative linear tendency in the general cortex, except the left temporal and occipital cortex, across the OH groups (Table 3).
Longitudinal influences of orthostatic hypotension groups
Seventy-seven PD patients were followed for a mean period of 24.2 ± 5.3 months (no-OH vs. delayed OH vs. classical OH: 23.3 ± 5.1 vs. 23.0 ± 4.8 vs. 25.7 ± 5.5; p = 0.117) and the longitudinal influences of the orthostatic BP drop are presented in Table 4 and Fig. 2. In this sub-analysis, age, disease duration and levodopa equivalent daily dose (LEDD) of the last trace point did not differ across the groups (age: p = 0.065; disease duration: p = 0.531; LEDD: p = 0.137). UPDRS Part II and MMSE score demonstrated progression during the follow-up period (time effects: p = 0.019, p = 0.008; respectively) and classical OH had worse outcomes on these scales (group effects: p = 0.006, p = 0.006; a = b < c, a > c; respectively). UPDRS total sum and Part III did not display any impairment during the follow-up (time effects: p = 0.075, p = 0.440; respectively), but maintained between-group differences (group effects: p = 0.004, p = 0.016; respectively). There were no interactions between time and group effects among the analyzed scales. When dopaminergic treatment effects were adjusted, UPDRS scores and MMSE did not worsen significantly over the follow-up period (time effect: p > 0.05). Classical OH similarly maintained its worsening trend on UPDRS total sum, Part II, Part III and MMSE scores (group effects: p = 0.012, p = 0.023, p = 0.034, p = 0.006; interaction: p = 0.574, p = 0.872, p = 0.346; p = 0.874; Supplementary Table 2 and Supplementary Fig. 1).
Discussion
In this study, ordinal subtypes of OH (delayed OH vs. classical OH) were compared to investigate the nature of OH in early PD. Group with classical OH manifested more severe clinical scales and biomarkers than other groups. However, no-OH and delayed OH groups did not differ significantly. The disparities had a linear gradient pattern across the OH spectrum. These findings were sustained during the longitudinal follow-up.
The population of this study was in the early stage of disease with non-demented mild PD. The similar clinical status reflects a relatively homogeneous pathophysiologic stage confined to the brainstem according to ‘bottom-up’ theory12,13. PD patients with classical OH were older than other groups, and the prevalence of classical OH was similar when compared to previous studies that estimated it in a range of 14–54%3.
The prevalence of delayed OH was also comparable11, but with a different clinical context. Gibbons and Freeman explored the natural history of delayed OH in a 10-year follow-up study and observed that about half progressed to classical OH. Among the subjects with delayed OH in initial testing who progressed to classical OH, >50% developed α-synucleinopathy. This study was not designed to investigate delayed OH in PD; therefore, it was difficult to infer any clinical significance in PD population. Our research is of value that it studied delayed OH in PD.
Motor related (dopaminergic) functions, including daily activities, were preferentially more affected in PD with classical OH when the covariates were controlled. Motor features of daily activities worsened over a 2-year period, in line with a previous study14. The differences among groups and aggravating linear trend of disease severity across groups were maintained throughout the follow-up period. The potential causes of the motor disparities could be the result of severer underlying disease burden and/or be attributed to fatigue or end-organ damage by blood pressure instability15,16.
Non-motor features were found to be more severe in PD with classical OH. Worse non-motor features could have affected adverse motor outcomes17. The participants showed increasing non-motor severity across the OH groups. The positive association of nondopaminergic parameters with ordinal OH further strengthens the argument that delayed OH is a mild form of neurogenic OH in early PD. The cognition of all three groups worsened with time. PD with classical OH progressed more aggressively while the worsening of no-OH and delayed OH were not disparate. This result is consistent with a previous study where OH is reported to impact cognitive decline5,18.
After adjusting for dopaminergic therapy, the cognitive and motor severities did not demonstrate significant worsening, regardless of the OH types. This was anticipatory because early non-demented PD (motor and cognitive aspects) responded to dopaminergic replacement19. However, classical OH had worse overall severities than the other groups, and its unfavorable outcomes were sustained during the follow-up period, independent of dopaminergic treatment. The observed baseline between-group differences and worsening tendencies across the OH spectrum during the follow-up period argues that different levels of orthostatic challenge may affect distinctive clinical progressions.
Association between OH and cognitive impairment has been discussed previously. In this cohort of early de novo PD, noradrenergic deficiency by locus coeruleus impairment and vascular theory of cerebral hypoperfusion could have attributed to the more rapid decline of cognition in classical OH20,21. Cortical thinning could also be a cause of such a finding.
Biomarkers of the cardiovascular system and cortical thickness depicted similar patterns across the groups as the clinical parameters. The frequency of disrupted circadian blood pressures, assessed by 24-hour ambulatory blood pressure monitoring, increased with the planned order of OH types. Patients with OH (both classical or delayed) were more aggressively deprived of cardiac sympathetic innervation than those with no-OH. Supine blood pressure tended to increase across the OH spectrum. The worsening gradient across the groups represented more damaged arterial baroreflex abnormalities1. Anterior dominant cortical thinning was worst in the classical OH. This was replicated in a previous study with hypotensive insults as a causative mechanism22,23,24,25. The cortex tended to become thinner across the OH groups which may result from different levels of hypotensive insults.
The strength of this study was that a large number of early drug-naïve PD were enrolled with extensive assessments encompassing non-motor and motor features. Other studies with comparable early PD involved small populations26,27. Only recently, a large scale prospective cohort was published however this performed limited evaluations and provided limited information3. PD is an age-dependent neurodegenerative disease in which clinical parameters are affected by aging. In this study, baseline characteristics with demonstrated differences were re-assessed with an adjustment of covariates, unlike in previous studies3. This study has several limitations. First, the enrolled patients did not go through every measurement due to the condition of each patient, and they were excluded from sub-analyses. In addition, many patients were lost during the follow-up. The large drop-outs are the major weakness because this would inevitably deepen selection bias. However, the study population included mild non-demented de novo PD to ensure reasonably homogenous pathologic stages and was followed for 2-year period on average. Its homogeneity of mild disease severity and relatively short follow-up duration may mitigate the bias that only the fitter patients could have been investigated. The large drop-outs could reduce the statistical power to discern group differences. On the contrary, it supports that achieved differences overcame the conservative null hypothesis produced by diminished statistical power; however, careful consideration is required in the interpretation since the drop-outs could also bias the alternative hypothesis. Second, autonomic evaluations were not comprehensive. Further evaluations of parasympathetic outflows could be of benefit in elucidating the pathophysiology of delayed OH. Third, patients had comorbid diseases, such as hypertension and diabetes mellitus, which contribute to autonomic disturbances. In addition, many subjects with comorbid diseases were on medications. We did not analyze the interactive influence of systemic diseases or drugs that might play a pathophysiologic role in autonomic failure. Finally, the follow-up duration was too short for definite conclusiveness. Large prolonged prospective studies with comprehensive evaluations of clinical aspects and biomarkers are required to further describe delayed OH.
In summary, not only the pattern of between-group differences but also the linear gradient across the ordinal subtypes of OH, and the maintenance of such traits with time suggest that delayed OH is a mild form of classical neurogenic OH in early PD, associated with less severe clinical burden and deterioration. This may facilitate patient selections in future neuroprotective studies.
Methods
Participants
This longitudinal study was approved by the Institutional Review at Seoul St. Mary’s Hospital, and all subjects provided written informed consent to participate. All experiments were performed in accordance with relevant guidelines and regulations. The study was registered (Identification Number: KCT0005552) in the Clinical Research Information Service (CRIS; http://cris.nih.go.kr), which is an online clinical trial registration system established by the Korea Centers for Disease Control and Prevention (KCDC) with support from the Korea Ministry of Health and Welfare (KMOHW) and embodied as a part of the Primary Registries in the World Health Organization (WHO) Registry Network.
Two hundred eighty-five drug-naïve patients newly diagnosed with PD between October 2014 and December 2019 were enrolled. The diagnosis of PD was based on the UK Parkinson’s Disease Society Brain Bank clinical diagnostic criteria28, and its diagnosis was substantiated by positron emission tomography imaging studies using 18F-N-(3-fluoropropyl)-2beta-carbon ethoxy-3beta-(4-iodophenyl) nortropane and 123I-metaiodobenzylguanidine (123I-MIBG) scintigraphy29,30. Baseline demographics such as age, sex, body mass index (BMI), disease duration, education, smoking status, and history of hypertension, diabetes mellitus, dyslipidemia, stroke, and coronary artery disease were investigated. Disease severity was evaluated with the UPDRS and modified H&Y stage. Global cognition was assessed by MMSE and CDR.
Patients with any of the following indications were excluded from the initial enrollment: (1) any symptoms or signs of atypical and/or secondary parkinsonism, (2) documentation of atrial fibrillation during head-up tilt electrocardiographic monitoring, (3) history of diabetic neuropathy, (4) history of peripheral arterial disease, (5) history of symptomatic stroke that might affect cognition and general performance, (6) taking medications such as tricyclic antidepressant and alpha-adrenergic antagonists that influence orthostatic challenge, (7) family history of dementia, and (8) clinical suspicion of dementia (CDR ≥ 1)31.
The global cognitive efficiency and disease severity of 77 patients with PD were re-evaluated by using MMSE and UPDRS, respectively (Fig. 1).
Questionnaires
Questionnaires were completed by 177 patients. Non-motor features, mood, quality of life, parasomnia, and symptoms related to orthostatic challenge were evaluated with the NMSS, MADRS, Parkinson’s Disease Quality of Life-39 (PDQ-39), RBDSQ and OHQ, respectively32,33,34,35,36. The sums of each questionnaire were used for analyses.
Head-up tilt test
All patients were at a full resting state before the exam. Continuous electrocardiograph leads and non-invasive blood pressure monitoring equipment were applied to the patients (YM6000, Mediana Tech, Redmond, WA, USA). A supine position was maintained for 20 min before tilting to 60° (Enraf-Nonius, Rotterdam, The Netherlands). While in the supine position, blood pressure and heart rate were measured every 5 min for 20 min. At the 60° position, blood pressure and heart rate were measured at 0, 3, 5, 10, 15, and 20 min. For analyses, the lowest tilt values for BP were chosen from 0 to 3 min for classical OH, and from 5 to 20 min for delayed OH. The first supine blood pressure (at 0 min) was excluded, and maximal supine systolic and diastolic blood pressures were selected among the measurements at 5, 10, 15, and 20 min. The lowest systolic and diastolic values at 0 or 3 min during the tilted position were chosen. The orthostatic blood pressure changes of systole (ΔSBP) and diastole (ΔDBP) were calculated. Patients were categorized as having classical OH or delayed OH if ΔSBP and/or ΔDBP ≥ 20/10 mmHg within 3 min or when the BP drops occurred after 5 min8. Cases that satisfied both classical OH and delayed OH criteria were categorized as classical OH.
Twenty-four-hour ambulatory blood pressure monitoring
Automated 24-hour blood pressure equipment (Mobil-O-Graph NG, IEM, Stolberg, Germany) was used to measure daytime and nighttime blood pressures from the upper arm. Blood pressures were recorded at 15-minute intervals throughout the day and 30-min intervals at night. Daytime was defined as a period between 8:00 a.m. and 23:59 p.m., and nighttime was from 00:00 a.m. to 07:59 a.m. Nocturnal hypertension was defined as increased absolute values of nighttime systolic and/or diastolic BP ≥ 120/70 mmHg37. Patients were classified as non-dipper if the ratio of night/day systolic and/or diastolic BP ≥ 138.
123I-metaiodobenzylguanidine scintigraphy
123I-MIBG scintigraphy was performed using a dual-head camera equipped with a low-energy high-resolution collimator (Siemens), and data were collected at 30-min (early) and 2-h (delayed) time points after a 111 MBq 123I-MIBG injection. A static image was obtained with a 128 × 128 matrix. Regions of interest were manually drawn around the heart and mediastinum. Tracer uptake was measured within each region of interest. For each time point, tracer uptake ratios of the H/M ratio were calculated and defined as early H/M (30 min) and delayed H/M (120 min). The lower limits of the reference value for early and delayed H/M ratios were set to be 1.70 and 1.78, respectively30.
Magnetic resonance imaging acquisition and cortical thickness measurements
The cortical thickness of 82 patients was analyzed by brain magnetic resonance imaging (MRI). A 3D T1-weighted magnetization-prepared rapid gradient-echo sequence was acquired using a 3-T scanner (Magnetom Verio, Siemens Healthcare, Erlangen, Germany) with a 12-channel head coil. Parameters were as follows: sagittal acquisition with FOV = 256 × 256 mm2; voxel size = 1 × 1 × 1 mm3; TR = 1780 msec; TE = 2.2 msec; flip angle = 9°; total acquisition time = 6 min 38 s. For the measurement of cortical thickness, we used the CIVET pipeline (http://mcin.ca/civet/) as described in detail elsewhere39,40. The native T1-weighted images of each subject were corrected for intensity inhomogeneity and spatially normalized to the MNI-152 template. After that, the images were tissue classified and hemispheric inner and outer cortical surfaces were automatically extracted using the constrained Laplacian-based automated segmentation with the proximities algorithm. Cortical thickness was measured by calculating the Euclidean distance between corresponding vertices on the gray matter/cerebrospinal fluid intersection surface and the white matter/gray matter boundary surface41. Diffusion smoothing with a 30-mm full width at half maximum kernel (FWHM) was used to increase the signal-to-noise ratio. To extract the mean regional cortical thickness, we used the Automated Anatomical Labeling (AAL) atlas template to define regional boundaries and averaged the cortical thickness of vertices within each of the regions of interest for each subject. Less than one third of the study population was included due to alterations of acquisition protocol and MRI equipment, and only patients with suitable uniformity for analyses were selected.
Statistical analysis
All statistical analyses were performed with jamovi software (version 1.2.16; retrieved from https://www.jamovi.org) and R software with additional car and emmeans packages (version 3.6.3; retrieved from https://cran.r-project.org) for Mac. Descriptive analyses and the analysis of variance or Kruskal–Wallis tests were applied to describe the baseline characteristics of PD patients. Categorical variables were analyzed by the χ2 test. Subgroups of PD patients were examined by analysis of covariance, adjusted for age, sex, disease duration, and additional covariates when needed, to investigate between-group differences. To discern any linear gradient in the order of no-OH, delayed OH, and classical OH, polynomial contrasts or Cochran-Armitage tests were used, as appropriate. In a sub-group analysis of PD patients, repeated measures analysis of variance was applied to measure the temporal patterns of PD subgroups. Multiple comparisons were corrected with a defined significance at a two-tailed p-value < 0.05.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Anonymized data generated during the current study are available from the corresponding author on reasonable request from individuals affiliated with research or health care institutions.
Code availability
Jamovi is a statistical spreadsheet and graphical user interface (GUI) for R. All the analyses, except Cochran-Armitage, were performed basically using jamovi software as mentioned in the method, statistical analysis section. The R packages mentioned were utilized within jamovi GUI.
References
Jain, S. & Goldstein, D. S. Cardiovascular dysautonomia in Parkinson disease: from pathophysiology to pathogenesis. Neurobiol. Dis. 46, 572–580 (2012).
Pont‐Sunyer, C. et al. The onset of nonmotor symptoms in Parkinson’s disease (The ONSET PD Study). Mov. Disord. 30, 229–237 (2015).
Hiorth, Y. H., Pedersen, K. F., Dalen, I., Tysnes, O. B. & Alves, G. Orthostatic hypotension in Parkinson disease: a 7-year prospective population-based study. Neurology 93, e1526–e1534 (2019).
Kim, J. S. et al. Association of cognitive dysfunction with neurocirculatory abnormalities in early Parkinson disease. Neurology 79, 1323–1331 (2012).
Anang, J. B. et al. Predictors of dementia in Parkinson disease: a prospective cohort study. Neurology 83, 1253–1260 (2014).
Fereshtehnejad, S. M. et al. New clinical subtypes of Parkinson disease and their longitudinal progression: a prospective cohort comparison with other phenotypes. JAMA Neurol. 72, 863–873 (2015).
De Pablo-Fernandez, E. et al. Association of autonomic dysfunction with disease progression and survival in Parkinson disease. JAMA Neurol. 74, 970–976 (2017).
Freeman, R. et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin. Auton. Res. 21, 69–72 (2011).
Brignole, M. et al. Practical instructions for the 2018 ESC guidelines for the diagnosis and management of syncope. Eur. Heart J. 39, e43–e80 (2018).
Gibbons, C. H. & Freeman, R. Delayed orthostatic hypotension: a frequent cause of orthostatic intolerance. Neurology 67, 28–32 (2006).
Gibbons, C. H. & Freeman, R. Clinical implications of delayed orthostatic hypotension: A 10-year follow-up study. Neurology 85, 1362–1367 (2015).
Braak, H. et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 24, 197–211 (2003).
Beach, T. G. et al. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 117, 613–634 (2009).
Kotagal, V., Lineback, C., Bohnen, N. I. & Albin, R. L. Orthostatic hypotension predicts motor decline in early Parkinson disease. Parkinsonism Relat. Disord. 32, 127–129 (2016).
Nakamura, T., Suzuki, M., Ueda, M., Hirayama, M. & Katsuno, M. Lower body mass index is associated with orthostatic hypotension in Parkinson’s disease. J. Neurol. Sci. 372, 14–18 (2017).
Kotagal, V., Szpara, A., Albin, R. L. & Bohnen, N. I. Fatigue in Parkinson’s disease associates with lower ambulatory diastolic blood pressure. J. Parkinsons Dis. 9, 575–581 (2019).
van der Heeden, J. F., Marinus, J., Martinez-Martin, P. & van Hilten, J. J. Importance of nondopaminergic features in evaluating disease severity of Parkinson disease. Neurology 82, 412–418 (2014).
Pilotto, A. et al. Orthostatic hypotension and REM sleep behaviour disorder: impact on clinical outcomes in alpha-synucleinopathies. J. Neurol. Neurosurg. Psychiatry 90, 1257–1263 (2019).
Kehagia, A. A., Baker, R. A. & Robbins, T. W. Cognitive impairment in Parkinson’s disease: the dual syndrome hypothesis. Neurodegener. Dis. 11, 79–92 (2013).
McDonald, C., Newton, J. L. & Burn, D. J. Orthostatic hypotension and cognitive impairment in Parkinson’s disease: causation or association? Mov. Disord. 31, 937–946 (2016).
Yoo, S. W. et al. Intervening effects of orthostatic blood pressure change on subcortical atrophy and cognition in de novo and drug-naïve Parkinson’s disease. J. Parkinsons Dis. 10, 153–160 (2020).
Shin, K. J. et al. Cortical morphology in patients with orthostatic intolerance. Eur. Neurol. 73, 264–270 (2015).
de la Torre, J. C. Critically attained threshold of cerebral hypoperfusion: the CATCH hypothesis of Alzheimer’s pathogenesis. Neurobiol. Aging 21, 331–342 (2000).
Jochemsen, H. M. et al. Blood pressure and progression of brain atrophy: the SMART-MR Study. JAMA Neurol. 70, 1046–1053 (2013).
Zonneveld, H. I. et al. The bidirectional association between reduced cerebral blood flow and brain atrophy in the general population. J. Cereb. Blood Flow. Metab. 35, 1882–1887 (2015).
Bonuccelli, U. et al. Orthostatic hypotension in de novo Parkinson disease. Arch. Neurol. 60, 1400–1404 (2003).
Bae, H. J., Cheon, S. M. & Kim, J. W. Orthostatic hypotension in drug-naive patients with Parkinson’s disease. J. Mov. Disord. 4, 33–37 (2011).
Gibb, W. & Lees, A. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 51, 745–752 (1988).
Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30, 1591–1601 (2015).
Ryu, D. W. et al. Initial versus follow-up sequential myocardial 123I-MIBG scintigraphy to discriminate Parkinson disease from atypical Parkinsonian syndromes. Clin. Nucl. Med. 44, 282–288 (2019).
Chin, J. et al. Re-standardization of the Korean-instrumental activities of daily living (K-IADL): Clinical usefulness for various neurodegenerative diseases. Dement. Neurocogn. Disord. 17, 11–22 (2018).
Koh, S. B. et al. Validation of the korean-version of the nonmotor symptoms scale for Parkinson’s disease. J. Clin. Neurol. 8, 276–283 (2012).
Ahn, Y. M. et al. A validation study of the Korean-version of the Montgomery-Asberg Depression Rating Scale. J. Korean Neuropsychiatr. Assoc. 44, 466–476 (2005).
Kwon, D. Y. et al. Translation and validation of the korean version of the 39-item Parkinson’s disease questionnaire. J. Clin. Neurol. 9, 26–31 (2013).
Lee, S. A., Paek, J. H., Han, S. H. & Ryu, H. U. The utility of a Korean version of the REM sleep behavior disorder screening questionnaire in patients with obstructive sleep apnea. J. Neurol. Sci. 358, 328–332 (2015).
Kaufmann, H., Malamut, R., Norcliffe-Kaufmann, L., Rosa, K. & Freeman, R. The orthostatic hypotension questionnaire (OHQ): validation of a novel symptom assessment scale. Clin. Auton. Res. 22, 79–90 (2012).
Williams, B. et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur. Heart J. 39, 3021–3104 (2018).
Parati, G. et al. European Society of hypertension practice guidelines for ambulatory blood pressure monitoring. J. Hypertens. 32, 1359–1366 (2014).
Shin, N. Y. et al. Different functional and microstructural changes depending on duration of mild cognitive impairment in Parkinson Disease. AJNR Am. J. Neuroradiol. 37, 897–903 (2016).
Shin, N. Y. et al. Retrosplenial cortical thinning as a possible major contributor for cognitive impairment in HIV patients. Eur. Radiol. 27, 4721–4729 (2017).
Kim, J. S. et al. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage 27, 210–221 (2005).
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2017R1D1A1B06028086).
Author information
Authors and Affiliations
Contributions
S.-W.Y. and J.-S.K. designed the study; S.-W.Y., J.-S.K., J.-Y.Y., and K.-S.L. carried out data collection; E.Y., U.Y., and N.-Y.S. analyzed the MRI data; S.-W.Y. and J.-S.K. analyzed the clinical data; S.-W.Y. drafted the manuscript; S.-W.Y., J.-S.K., J.-Y.Y., E.Y., U.Y., N.-Y.S., and K.-S.L. revised the manuscript. J.-S.K. obtained funding. All authors read and approved the final version for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yoo, SW., Kim, JS., Yoo, JY. et al. Delayed orthostatic hypotension in Parkinson’s disease. npj Parkinsons Dis. 7, 37 (2021). https://doi.org/10.1038/s41531-021-00181-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-021-00181-y
This article is cited by
-
Estimating motor progression trajectory pursuant to temporal dynamic status of cardiac denervation in Parkinson’s disease
Journal of Neurology (2024)
-
A 3-year natural history of orthostatic blood pressure dysregulation in early Parkinson’s disease
npj Parkinson's Disease (2023)
-
Caudate-anchored cognitive connectivity pursuant to orthostatic hypotension in early Parkinson's disease
Scientific Reports (2022)
-
Corneal confocal microscopy differentiates patients with Parkinson’s disease with and without autonomic involvement
npj Parkinson's Disease (2022)