Abstract
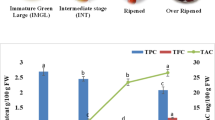
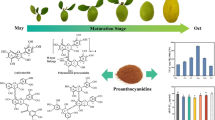
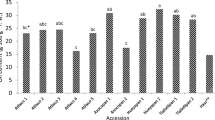
It is important to determine the most suitable ripening period of fruit regarding bioactive compounds for evaluating as an industrial and in human nutrition. The aims of this study were to determine the changes in non-anthocyanin phenolics, anthocyanins, and antioxidant activity of Mahonia aquifolium fruit throughout ripening and to determine the biosynthetic pathway of the phenolic compounds. The fruit was collected from shrubs at 10 different ripening stages between June 21 and August 17, 2016. The samples were analyzed for individual anthocyanins, non-anthocyanin phenolics, and antioxidant activity. There were significant decreases in gentisic, chlorogenic, p-coumaric, ferulic acids, rutin, and isorhamnetin 3-O-glucoside and increases in protocatechuic, syringic acid, caffeic acid, myricetin, and luteolin. The concentrations of delphinidin-3-O-glucoside, cyanidin-3-O-glucoside, and pelargonidin-3-O-glucoside rose progressively from the beginning to the end of ripening, while decreases occurred from the 6th stage in the amounts of malvidin-3-O-glucoside, peonidin-3-O-glucoside, cyanidin-3-O-rutinoside, and delphinidin-3-O-rutinoside. A decrease followed by an increase in the antioxidant activity indicated that the phenolic profile of the fruit changed toward the end of ripening, including for the compounds with higher antioxidant potential. In this study, the most suitable harvesting period for Mahonia berries was determined


Similar content being viewed by others
References
Alberti A, Zielinski AAF, Couto M, Judacewski P, Mafra LI, Nogueira A (2017) Distribution of phenolic compounds and antioxidant capacity in apples tissues during ripening. J Food Sci Technol 6:1511–1518
Aseel DG, Rashad YM, Hammad SM (2019) Arbuscular Mycorrhizal fungi trigger transcriptional expression of flavonoid and chlorogenic acid biosynthetic pathways genes in tomato against tomato mosaic virus. Sci Rep 1:1–10
Belwal T, Pandey A, Bhatt ID, Rawal RS, Luo Z (2019) Trends of polyphenolics and anthocyanins accumulation along ripening stages of wild edible fruits of Indian Himalayan region. Sci Rep 1:5894
Benzie IF, Strain J (1999) Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. In: Packer L (ed) Methods in enzymology. Elsevier, San Diego, pp 15–27
Biała W, Jasiński M (2018) The Phenylpropanoid case—it is transport that matters. Front Plant Sci 9:1–9
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free-radical method to evaluate antioxidant activity. Lebenson Wiss Technol 1:25–30
Butkhup L, Samappito S (2011) Changes in physico-chemical properties, polyphenol compounds and antiradical activity during development and ripening of maoluang (Antidesma bunius L. Spreng) fruits. J Fruit Ornam Plant Res 1:85–99
Cao G, Sofic E, Prior RL (1997) Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free Radic Biol Med 5:749–760
Casañal A, Zander U, Muñoz C, Dupeux F, Luque I, Botella MA et al (2013) The strawberry pathogenesis-related 10 (PR-10) Fra a proteins control flavonoid biosynthesis by binding to metabolic intermediates. J Biol Chem 49:35322–35332
Celli GB, Pereira-Netto AB, Beta T (2011) Comparative analysis of total phenolic content, antioxidant activity, and flavonoids profile of fruits from two varieties of Brazilian cherry (Eugenia uniflora L.) throughout the fruit developmental stages. Food Res Int 8:2442–2451
Chaves-Silva S, dos Santos AL, Chalfun-Júnior A, Zhao J, Peres LE, Benedito VA (2018) Understanding the genetic regulation of anthocyanin biosynthesis in plants—tools for breeding purple varieties of fruits and vegetables. Phytochemistry 153:11–27
Coklar H, Akbulut M (2017) Anthocyanins and phenolic compounds of Mahonia aquifolium berries and their contributions to antioxidant activity. J Funct Foods 35:166–174
Craft BD, Kerrihard AL, Amarowicz R, Pegg RB (2012) Phenol-based antioxidants and the in vitro methods used for their assessment. Compr Rev Food Sci 2:148–173
de Simón BF, Hernández T, Estrella I (1992) Relationship between chemical structure and biosynthesis and accumulation of certain phenolic compounds in grape skins during ripening. Z Lebensm Unters Forsch 2:124–128
Deng Y, Lu S (2017) Biosynthesis and regulation of phenylpropanoids in plants. Crit Rev Plant Sci 4:257–290
Dong T, Han R, Yu J, Zhu M, Zhang Y, Gong Y et al (2019) Anthocyanins accumulation and molecular analysis of correlated genes by metabolome and transcriptome in green and purple asparaguses (Asparagus officinalis, L.). Food Chem 271:18–28
El Sayed Bashandy H (2016) Flavonoid Metabolomics in Gerbera hybrida and Elucidation of Complexity in the Flavonoid Biosynthetic Pathway (2016). https://helda.helsinki.fi/bitstream/handle/10138/159523/flavonoi.pdf?sequence=1. Accessed 09 September 2019
Falcone Ferreyra ML, Rius S, Casati P (2012) Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front Plant Sci 3:222
Gibson L, Rupasinghe H, Forney C, Eaton L (2013) Characterization of changes in polyphenols, antioxidant capacity and physico-chemical parameters during lowbush blueberry fruit ripening. Antioxidants 4:216–229
Gutha LR, Casassa LF, Harbertson JF, Naidu RA (2010) Modulation of flavonoid biosynthetic pathway genes and anthocyanins due to virus infection in grapevine (Vitis vinifera L.) leaves. BMC Plant Biol 1:187
Heo HJ, Kim YJ, Chung D, Kim D-O (2007) Antioxidant capacities of individual and combined phenolics in a model system. Food Chem 1:87–92
Jaakola L (2013) New insights into the regulation of anthocyanin biosynthesis in fruits. Trend Plant Sci 9:477–483
Jordão A, Da Silva JR, Laureano O (2015) Evolution of anthocyanins during grape maturation of two varieties (Vitis vinifera L.). Castelao Frances Touriga Francesa Vitis 2:93–94
MSTAT Development Team (1989) MSTAT user’s guide: a microcomputer program for the design management and analysis of agronomic research experiments. Michigan State University, East Lansing, MI
Meighani H, Ghasemnezhad M, Bakhshi D (2017) An evaluation of the phytochemical properties of some pomegranate cultivars during fruit development and ripening. Int J Hortic 2:193–204
Murakami M, Yamaguchi T, Takamura H, Matoba T (2003) Effects of ascorbic acid and α-tocopherol on antioxidant activity of polyphenolic compounds. J Food Sci 5:1622–1625
Prasanna V, Prabha T, Tharanathan R (2007) Fruit ripening phenomena—an overview. Crit Rev Food Sci Nutr 1:1–19
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 9–10:1231–1237
Rehman RNU, You YH, Ali S, Wang YL, Zhang L, Li PM et al (2018) Phenolic compounds as biochemical markers of senescence in woody ornamental flowers of Malus crabapple. Hortic Environ Biotechnol 1:1–10
Ribera A, Reyes-Diaz M, Alberdi M, Zuñiga G, Mora M (2010) Antioxidant compounds in skin and pulp of fruits change among genotypes and maturity stages in highbush blueberry (Vaccinium corymbosum L.) grown in southern Chile. J Soil Sci Plant Nutr 4:509–536
Rice-Evans CA, Miller NJ, Paganga G (1996) Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 7:933–956
Rice-Evans C, Miller N, Paganga G (1997) Antioxidant properties of phenolic compounds. Trends Plant Sci 4:152–159
Shahidi F, Ambigaipalan P (2015) Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—a review. J Funct Foods 18:820–897
Sharma A, Shahzad B, Rehman A, Bhardwaj R, Landi M, Zheng B (2019) Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 13:2452
Somani SJ, Modi KP, Majumdar AS, Sadarani BN (2015) Phytochemicals and their potential usefulness in inflammatory bowel disease. Phytother Res 3:339–350
Soobrattee MA, Neergheen VS, Luximon-Ramma A, Aruoma OI, Bahorun T (2005) Phenolics as potential antioxidant therapeutic agents: mechanism and actions. Mutat Res Fund Mol Mech Mut 1–2:200–213
Yeh C-T, Yen G-C (2003) Effects of phenolic acids on human phenolsulfotransferases in relation to their antioxidant activity. J Agric Food Chem 5:1474–1479
Yin R, Messner B, Faus-Kessler T, Hoffmann T, Schwab W, Hajirezaei M-R et al (2012) Feedback inhibition of the general phenylpropanoid and flavonol biosynthetic pathways upon a compromised flavonol-3-O-glycosylation. J Exp Bot 7:2465–2478
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Hacer Coklar and Mehmet Akbulut performed the experiments, developing the methodology and preparing the manuscript and laboratory studies.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no confict of interest.
Additional information
Communicated by Ali Sarkhosh.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Coklar, H., Akbulut, M. Changes in phenolic acids, flavonoids, anthocyanins, and antioxidant activities of Mahonia aquifolium berries during fruit development and elucidation of the phenolic biosynthetic pathway. Hortic. Environ. Biotechnol. 62, 785–794 (2021). https://doi.org/10.1007/s13580-021-00348-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-021-00348-9