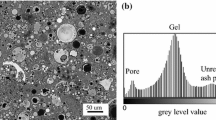
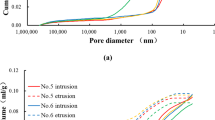
Abstract
The pore structure of alkali-activated fly ash mortar (AAFAM) under different mix ratios and curing system was measured by mercury intrusion porosimetry (MIP) and scanning electron microscopy (SEM). Based on the fractal theory, the integral shape dimension of the pore surface of the AAFAM is obtained and discussed its relationship with total porosity, proportion of gel pores, transition pore, capillary pores and macropores, average pore diameter, median pore diameter, pore surface area, pore size distribution, and the influence of mass ratio of alkali activators, curing temperature, and curing age on pore structures. In addition, data regression analysis (DRA) is a statistical tool and applied to study the relationship between microscopic pore parameters and the fractal dimension of the AAFAM mixtures. The results show that the fractal dimension of the AAFAM is between 2.6 and 2.9, and the pore size distribution has a linear relationship with the fractal dimension, and the internal pore diameter of the AAFAM will gradually decrease and AAFAM’s matrix gradually becomes denser and the microstructure integrity is more stable as the curing age and temperature increases, and the mechanical strength will be higher. The AAFAM mixture composed of Na2SiO3/NAOH with a mass ratio of 1.0 and 100% fly ash is considered the most suitable mixture. AAFAM can make full use of industrial by-products (fly ash) and seems to be a suitable candidate for green environmental protection materials for engineering applications.













Similar content being viewed by others
References
Juenger, M.C.G.; Winnefeld, F.; Provis, J.L.; Ideker, J.: Advances in alternative cementitious binders. Cem. Concr. Res. 41, 1232–1243 (2011). https://doi.org/10.1016/j.cemconres.2010.11.012
Provis, J.L.; van Deventer, J.S.J.: Geopolymers: Structures, Processing, Properties and Industrial Applications, p. 448. Woodhead, Cambridge, UK (2009)
Duxson, P.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J.: The role of inorganic polymer technology in the development of ‘green concrete.’ Cem. Concr. Res. 37, 1590–1597 (2007). https://doi.org/10.1016/j.cemconres.2007.08.018
Shekhovtsova, J.; Kovtun, M.; Kearsley, E.P.: Evaluation of short-and long-term properties of heat-cured alkali-activated fly ash concrete. Mag. Concr. Res. 67(16), 897–905 (2015)
Cao, R.; Li, B.; You, N.; Zhang, Y.; Zhang, Z.: Properties of alkali-activated ground granulated blast furnace slag blended with ferronickel slag. Constr. Build. Mater. 192, 123–132 (2018). https://doi.org/10.1016/j.conbuildmat.2018.10.112
You, N.; Li, B.; Cao, R.; Shi, J.; Chen, C.; Zhang, Y.: The influence of steel slag and ferronickel slag on the properties of alkali-activated slag mortar. Constr. Build. Mater. 227, 116614 (2019). https://doi.org/10.1016/j.conbuildmat.2019.07.340
Provis, J.L.; Duxson, P.; van Deventer, J.S.J.: The role of particle technology in developing sustainable construction materials. Adv. Powder Technol. 21, 2–7 (2010). https://doi.org/10.1016/j.apt.2009.10.006
Khan, M.S.H.; Kayali, O.: Chloride binding ability and the onset corrosion threat on alkali-activated GGBFS and binary blend pastes. Eur J Environ Civ Eng 22(8), 1023–1039 (2018)
Gao, X.; Yu, Q.L.; Brouwers, H.J.H.: Assessing the porosity and shrinkage of alkali-activated slag-fly ash composites designed applying a packing model. Construct. Build. Mater. 119, 175–184 (2016)
Lee, N.K.; Lee, H.K.: Setting and mechanical properties of alkali-activated fly ash/slag concrete manufactured at room temperature. Construct. Build. Mater. 47, 1201–1209 (2013)
Bakharev, T.: Geopolymeric materials prepared using Class F fly ash and elevated temperature curing. Cement Concr. Res. 35(6), 1224–1232 (2005)
Bakharev, T.: Geopolymeric materials prepared using Class F fly ash and elevated temperature curing. Cem. Concr. Res. 35(6), 1224–1232 (2005). https://doi.org/10.1016/j.cemconres.2004.06.031
Ryu, G.S.; Lee, Y.B.; Koh, K.T.; Chung, Y.S.: The mechanical properties of fly ash-based geopolymer concrete with alkaline activators. Construct. Build. Mater. 47, 409–418 (2013)
Sarker, P.K.: Bond strength of reinforcing steel embedded in fly ash-based geopolymer concrete. Mater. Struct. 44(5), 1021–1030 (2011)
Oh, J.E.; Moon, J.; Oh, S.-G.; Clark, S.M.; Monteiro, P.J.: Microstructural and compositional change of NaOH-activated high calcium fly ash by incorporating Na-aluminate and co-existence of geopolymeric gel and C-S–H (I). Cem. Concr. Res. 42(5), 673–685 (2012)
Ahmaruzzaman, M.: A review on the utilization of fly ash. Progr. Energy. Comb. Sci. 36, 327–363 (2010)
Duxson, P.; Provis, J.L.: Designing precursors for geopolymer cements. J. Am. Ceram. Soc. 91, 3864–3869 (2008)
Zhang, Z.; Zhu, H.; Zhou, C.H.; Wang, H.: Geopolymer from kaolin in China: an overview. Appl. Clay Sci. 119, 31–41 (2016)
Ma, Y.; Hu, J.; Ye, G.: The pore structure and permeability of alkali activated fly ash. Fuel 104, 771–780 (2013)
Bernal, S.A.; Provis, J.L.; Rose, V.; De Guitiérrez, R.M.: Evolution of binder structure in sodium silicate-activated slag-metakaolin blends. Cem. Concr. Compos. 33, 46–54 (2011)
Sagoe-Crentsil, K.; Weng, L.: Dissolution process, hydrolysis and condensation reactions during geopolymer synthesis: Part II. High Si/Al ratio systems. J. Mater. Sci. 42, 3007–3014 (2007)
Duxson, P.; Mallicoat, S.W.; Lukey, G.C.; Krivem, W.M.; Van Deventer, J.S.J.: The effect of alkali and Si/Al ratio on the development of mechanical properties of metakaolin-based geopolymers. Colloids Surf. A 292, 8–20 (2007)
Duan, P.; Yan, C.; Zhou, W.; Luo, W.; Shen, C.: An investigation of the microstructure and durability of a fluidized bed fly ash-metakaolin geopolymer after heat and acid exposure. Mater. Des. 74, 125–137 (2015)
Lloyd, R.R.; Provis, J.L.; Smeaton, K.J.; Van Deventer, J.S.J.: Spatial distribution of pores in fly ash-based inorganic polymer gels visualized by Wood’s metal intrusion. Microporous Mesoporous Mater. 126, 32–39 (2009)
Provis, J.L.; Myers, R.J.; White, C.E.; Rose, V.; Van Deventer, J.S.J.: X-ray microtomography shows pore structure and tortuosity in alkali-activated binders. Cem. Concr. Res. 42, 855–864 (2012)
Zhang, Z.; Wang, H.; Zhu, Y.; Reid, A.; Provis, J.L.; Bullen, F.: Using fly ash to partially substitute metakaolin in geopolymer synthesis. Appl. Clay Sci. 88–89, 194–201 (2014)
Mangat, P.; Lambert, P.: Sustainability of alkali-activated cementitious materials and geopolymers, in: Sustain. Constr. Mater., Elsevier Ltd, 2016, 459–476https://doi.org/10.1016/B978-0-08-100370-1.00018-4.
Neville, A.M.: Properties of Concrete. Pearson Education Limited, London (2011)
Kumar, R.; Bhattacharjee, B.: Assessment of permeation quality of concrete through mercury intrusion porosimetry. Cem. Concr. Res. 34, 321–328 (2004). https://doi.org/10.1016/j.cemconres.2003.08.013
Jin, H.; Liu, J.; Jiang, Z.; Zhou, H.; Liu, J.: Influence of the rainfall intensity on the chloride ion distribution in concrete with different levels of initial water saturation. Constr. Build. Mater. 281, 122561 (2021). https://doi.org/10.1016/j.conbuildmat.2021.122561
Sidney Mindess, D.; Francis Young, J.; Darwin, Concrete, Prentice Hall, Pearson Education, Inc., Upper Saddle River, NJ 07458, U.S.A., 2003.
Li, Y.; Li, J.: Capillary tension theory for prediction of early autogenous shrinkage of self-consolidating concrete. Constr. Build. Mater. 53, 511–516 (2014). https://doi.org/10.1016/j.conbuildmat.2013.12.010
Petermann, J.C.; Saeed, A.; Hammons, M. I.; Alkali-activated geopolymers: a literature review air force research laboratory materials and manufacturing directorate, 2010.
Medina-Serna, T.; Arredondo-Rea, S.; Gómez-Soberón, J.; Rosas-Casarez, C.; Corral-Higuera, R.: Effect of curing temperature in the alkali-activated blast-furnace slag paste and their structural influence of porosity. Adv. Sci. Technol. Res. J. 10, 74–79 (2016)
Fang, G.; Ho, W.K.; Tu, W.; Zhang, M.: Workability and mechanical properties of alkali-activated fly ash-slag concrete cured at ambient temperature. Constr. Build. Mater. 172, 476–487 (2018). https://doi.org/10.1016/j.conbuildmat.2018.04.008
Cwirzen, A.; Engblom, R.; Punkki, J.; Habermehl-Cwirzen, K.: Effects of curing: comparison of optimised alkali-activated PC-FA-BFS and PC concretes. Mag. Concr. Res. 66, 315–323 (2014). https://doi.org/10.1680/macr.13.00231
Olalekan, O.; Ojedokun, Pal.; Mangat, S.: Chloride diffusion in alkali activated concrete, II Int. Conf. Concr. Sustain. (2016) 521–531.
Zhang, B.; Li, S.: Determination of the surface fractal dimension for porous media by mercury porosimetry. Ind. Eng. Chem. Res. 34(4), 1383–1386 (1995)
Ji, X.; Chan, S.; Feng, N.: Fractal model for simulating the space-filling process of cement hydrates and fractal dimensions of pore structure of cement-based materials. Cem. Concr. Res. 27(11), 1691–1699 (1997)
Xingye, Li.: Research on performance of alkali-activated fly ash mortar [D]. Southwest Jiaotong University, Chengdu (2018).
Fu, V.; Beaudoin, J.J.: On the distinction between delayed and secondary ettringite formation in concrete. Cem. Concr. Res. 26, 979–980 (1996). https://doi.org/10.1016/0008-8846(96)00080-4
Acknowledgements
The work described in this paper was fully supported by grants from the Sichuan Science and Technology Program (2019YFG0001) and Sichuan Provincial Department of Science and Technology Project (2020ZYD011).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Rights and permissions
About this article
Cite this article
Jin, H., Li, F. & Li, X. Research on the Micro-Pore Structures of AAFAM. Arab J Sci Eng 46, 10885–10900 (2021). https://doi.org/10.1007/s13369-021-05557-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-021-05557-z