Abstract
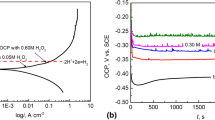
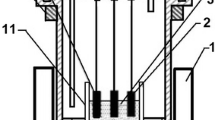
The formation of a rust layer on iron and steels surfaces accelerates their degradation and eventually causes material failure. In addition to fabricating a protective layer or using a sacrificial anode, repairing or removing the rust layer is another way to reduce the corrosion rate and extend the lifespans of iron and steels. Herein, an electrochemical healing approach was employed to repair the rust layer in molten Na2CO3-K2CO3. The rusty layers on iron rods and screws were electrochemically converted to iron in only several minutes and a metallic luster appeared. Scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDS) analyses showed that the structures of the rust layer after healing were slightly porous and the oxygen content reached a very low level. Thus, high-temperature molten-salt electrolysis may be an effective way to metalize iron rust of various shapes and structures in a short time, and could be used in the repair of cultural relics and even preparing a three-dimensional porous structures for other applications.
Similar content being viewed by others
References
J. de Beer, E. Worrell, and K. Blok, Future technologies for energy-efficient iron and steel making, Annu. Rev. Energy Env., 23(1998), No. 1, p. 123.
S.J. Oh, D.C. Cook, and H.E. Townsend, Characterization of iron oxides commonly formed as corrosion products on steel, Hyperfine Interact., 112(1998), No. 1–4, p. 59.
H. Tamura, The role of rusts in corrosion and corrosion protection of iron and steel, Corros. Sci., 50(2008), No. 7, p. 1872.
Z.M. Wang, X.Y. Zeng, and W.L. Huang, Parameters and surface performance of laser removal of rust layer on A3 steel, Surf. Coat. Technol., 166(2003), No. 1, p. 10.
Z.L. Tang, A review of corrosion inhibitors for rust preventative fluids, Curr. Opin. Solid State Mater. Sci., 23(2019), No. 4, art. No. 100759.
V. Narayanan, R.K. Singh, and D. Marla, Laser cleaning for rust removal on mild steel: An experimental study on surface characteristics, [in] The 3rd International Conference on Design and Manufacturing Engineering, Vol. 221, 2018, Melbourne, p. 01007.
A. Azhari, C. Schindler, K. Hilbert, C. Godard, and E. Kerscher, Influence of waterjet peening and smoothing on the material surface and properties of stainless steel 304, Surf. Coat. Technol., 258(2014), p. 1176.
G.Z. Chen, D.J. Fray, and T.W. Farthing, Direct electrochemical reduction of titanium dioxide to titanium in molten calcium chloride, Nature, 407(2000), No. 6802, p. 361.
A.M. Abdelkader, K.T. Kilby, A. Cox, and D.J. Fray, Voltam-metry of electro-deoxidation of solid oxides, Chem. Rev., 113(2013), No. 5, p. 2863.
S.L. Wang and Y.J. Li, Reaction mechanism of direct electro-reduction of titanium dioxide in molten calcium chloride, J. Electroanal. Chem., 571(2004), No. 1, p. 37.
A. Allanore, L. Yin, and D.R. Sadoway, A new anode material for oxygen evolution in molten oxide electrolysis, Nature, 497(2013), No. 7449, p. 353.
T. Wang, H.P. Gao, X.B. Jin, H.L. Chen, J.J. Peng, and G.Z. Chen, Electrolysis of solid metal sulfide to metal and sulfur in molten NaCl-KCl, Electrochem. Commun., 13(2011), No. 12, p. 1492.
H.Y. Yin, B. Chung, and D.R. Sadoway, Electrolysis of a molten semiconductor, Nat. Commun., 7(2016), art. No. 12584.
J.K. Qu, H.W. Xie, Q.S. Song, Z.Q. Ning, H.J. Zhao, and H.Y. Yin, Electrochemical desulfurization of solid copper sulfides in strongly alkaline solutions, Electrochem. Commun., 92(2018), p. 14.
S.Q. Jiao and H.M. Zhu, Novel metallurgical process for titanium production, J. Mater. Res., 21(2006), No. 9, p. 2172.
R.O. Suzuki, M. Aizawa, and K. Ono, Calcium-deoxidation of niobium and titanium in Ca-saturated CaCl2 molten salt, J. Alloys Compd., 288(1999), No. 1–2, p. 173.
D. Hu, A. Dolganov, M.C. Ma, B. Bhattacharya, M.T. Bishop, and G.Z. Chen, Development of the Fray-Farthing-Chen cam-bridge process: towards the sustainable production of titanium and its alloys, JOM, 70(2018), No. 2, p. 129.
H.W. Xie, H.J. Zhao, Q.S. Song, Z.Q. Ning, J.K. Qu, and H.Y. Yin, Anodic gases generated on a carbon electrode in oxide-ion containing molten CaCl2 for the electro-deoxidation process, J. Electrochem. Soc, 165(2018), No. 14, p. E759.
D.H. Wang, A.J. Gmitter, and D.R. Sadoway, Production of oxygen gas and liquid metal by electrochemical decomposition of molten iron oxide, J. Electrochem. Soc., 158(2011), No. 6, p. E51.
N.J. Siambun, H. Mohamed, D. Hu, D. Jewell, Y.K. Beng, and G.Z. Chen, Utilisation of carbon dioxide for electro-carburisa-tion of mild steel in molten carbonate salts, J. Electrochem. Soc., 158(2011), No. 11, p. H1117.
W. Xiao, X.B. Jin, Y. Deng, D.H. Wang, and G.Z. Chen, Three-phase interlines electrochemically driven into insulator compounds: A penetration model and its verification by electrore-duction of solid AgCl, Chem. Eur. J., 13(2007), No. 2, p. 604.
W. Xiao, X.B. Jin, Y. Deng, D.H. Wang, and G.Z. Chen, Rationalisation and optimisation of solid state electro-reduction of SiO2 to Si in molten CaCl2 in accordance with dynamic three-phase interlines based voltammetry, J. Electroanal. Chem., 639(2010), No. 1–2, p. 130.
W. Xiao, X.B. Jin, Y. Deng, D.H. Wang, X.H. Hu, and G.Z. Chen, Electrochemically driven three-phase interlines into insulator compounds: Electroreduction of solid SiO2 in molten CaCl2, Chem. Phys. Chem., 7(2006), No. 8, p. 1750.
E. Gordo, G.Z. Chen, and D.J. Fray, Toward optimisation of electrolytic reduction of solid chromium oxide to chromium powder in molten chloride salts, Electrochim. Acta, 49(2004), No. 13, p. 2195.
W. Xiao and D.H. Wang, The electrochemical reduction processes of solid compounds in high temperature molten salts, Chem. Soc. Rev., 43(2014), No. 10, p. 3215.
X.H. Cheng, L. Fan, H.Y. Yin, H. Liu, K.F. Du, and D.H. Wang, High-temperature oxidation behavior of Ni-11Fe-10Cu alloy: Growth of a protective oxide scale, Corros. Sci., 112(2016), p. 54.
X.H. Cheng, H.Y. Yin, and D.H. Wang, Rearrangement of oxide scale on Ni-11Fe-10Cu alloy under anodic polarization in molten Na2CO3-K2CO3, Corros. Sci., 141(2018), p. 168.
H.Y. Yin, D.Y. Tang, D. Zhu, Y. Zhang, and D.H. Wang, Production of iron and oxygen in molten K2CO3-Na2CO3 by electrochemically splitting Fe2O3 using a cost affordable inert anode, Electrochem. Commun., 13(2011), No. 12, p. 1521.
D.Y. Tang, K.Y. Zheng, H.Y. Yin, X.H. Mao, D.R. Sadoway, and D.H. Wang, Electrochemical growth of a corrosion-resistant multi-layer scale to enable an oxygen-evolution inert anode in molten carbonate, Electrochim. Acta, 279(2018), p. 250.
X.H. Cheng, D.D. Tang, H. Zhu, and D.H. Wang, Cobalt powder production by electro-reduction of Co3O4 granules in molten carbonates using an inert anode, J. Electrochem. Soc., 162(2015), No. 6, p. E68.
D.Y. Tang, H.Y. Yin, W. Xiao, H. Zhu, X.H. Mao, and D.H. Wang, Reduction mechanism and carbon content investigation for electrolytic production of iron from solid Fe2O3 in molten K2CO3-Na2CO3 using an inert anode, J. Electroanal. Chem., 689(2013), p. 109.
D.Y. Tang, H.Y. Yin, X.H. Cheng, W. Xiao, and D.H. Wang, Green production of nickel powder by electroreduction of NiO in molten Na2CO3-K2CO3, Int. J. Hydrogen Energy, 41(2016), No. 41, p. 18699.
A. Allanore, H. Lavelaine, G. Valentin, J.P. Birat, and F.M. Lapicque, Iron metal production by bulk electrolysis of iron ore particles in aqueous media, J. Electrochem. Soc., 155(2008), No. 9, p. E125.
A. Cox and D.J. Fray, Electrolytic formation of iron from haematite in molten sodium hydroxide, Ironmaking Steelmaking, 35(2008), No. 8, p. 561.
D.H. Wang, X.B. Jin, and G.Z. Chen, Solid state reactions: An electrochemical approach in molten salts, Annu. Rep. Prog. Chem. Sect. C, 104(2008), p. 189.
F. Lantelme and H. Groult, Molten Salts Chemistry: From Lab to Applications, vol. 28, Elsevier, Amsterdam, 2013.
N. Linares, A.M. Silvestre-Albero, E. Serrano, J. Silvestre-Albero, and J. García-Martínez, Mesoporous materials for clean energy technologies, Chem. Soc. Rev., 43(2014), No. 22, p. 7681.
M.Y. Wang, X.T. Yu, Z. Wang, X.Z. Gong, Z.C. Guo, and L. Dai, Hierarchically 3D porous films electrochemically constructed on gas-liquid-solid three-phase interface for energy application, J. Mater. Chem. A, 5(2017), No. 20, p. 9488.
Acknowledgements
This work is financially supported by the Fundamental Research Funds for the Central Universities (No. N172505002), the National Natural Science Foundation of China (No. 51704060), the National Thousand Youth Talent Program of China, and the 111 Project (No. B16009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Dy., Ma, X., Xie, Hw. et al. Electrochemical derusting in molten Na2CO3-K2CO3. Int J Miner Metall Mater 28, 637–643 (2021). https://doi.org/10.1007/s12613-020-2068-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12613-020-2068-2