Abstract
Hydrogeochemical processes responsible for groundwater composition within the Asutifi-North district were assessed. The aim was to delineate the factors influencing groundwater within the district. Results show that the pH values of groundwater within the district are moderately acidic to neutral and ranged from 4.4 to 7.5 with a mean value and standard deviation of 6.1 ± 0.7 pH units. 67.4% of groundwater is acidic (pH 4–6.5) due principally to the influence of aquifer materials on the chemistry of groundwater. Acid mine drainage investigations in groundwater within the district shows that, when exposed, sulphides such as pyrites (FeS2) and arsenopyrite (FeAsS) react with water and oxygen to principally produce Fe(OH)3. The major ion concentrations are within the WHO (2004) Guideline values for drinking water. The relative abundance of cations and anions in groundwater within the district is in the order of: Ca2+ > Na+ > Mg2+ > K+ and HCO3− > Cl− > SO42−, respectively. The study further shows that, albite/anorthite-(plagioclase) and calcite/dolomite(carbonates) dissolution as well as ion-exchange reactions may have contributed significantly to major ion concentrations in groundwater within the district. Hydrochemical facies using Piper trillinear diagrams delineated two major water types, the Ca–Mg–HCO3 and Na–Cl water types, with Ca–Mg–Cl, Ca–Mg–SO4–Cl and Na–K–HCO3 as minor water types. Using principal component analysis (PCA), three (3) main principal components accounting for 73.1% of the total variance with eigenvalues > 1 were extracted. PCA delineated the main natural processes through which groundwater within the district acquires its chemical characteristics, the incongruent dissolution of silicate/aluminosilicates and the prevalence of pollution principally from the application of inorganic fertilizers such as nitrates and ammonia via agricultural activities within the district.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Groundwater is an integral part within the framework of potable water supply to most communities and provides the water needs of millions of people who hitherto were without access to potable water across the world. According to the statistics, nearly one-third of the world’s population depends on groundwater for drinking (Tay 2015). Notwithstanding, nearly 30% of freshwater in the world is stored as groundwater with almost 97% of all freshwater readily available for use (Tay 2015). The percentage freshwater (30%) availability, however, is threatened by natural biochemical and geochemical processes within aquifers as well as anthropogenic activities. The monitoring of these threats is therefore critical to ensure the protection of available freshwater for human use throughout the world. Within the framework of the monitoring of available freshwater, groundwater monitoring plays a critical role. Knowledge of groundwater chemistry is not only critical for ascertaining the source of groundwater contamination but also the identification and characterization of the factors controlling the basic hydrochemistry in a region (Surinaidu 2016). The application of geochemical footprint in the evaluation of various hydrogeochemical processes such as water–rock interaction, identification of recharge area, ion-exchange processes along groundwater flow paths, mechanisms controlling the chemistry of groundwater in a region, salinization of groundwaters in coastal aquifers and the stability of silicate/carbonate minerals and their possible secondary weathering products has been extensively studied (Tardy 1971; Jankowski et al. 1998; Andre et al. 2005; Singh et al. 2008; Hussien et al. 2016; Surinaidu et al. 2016; Tay et al. 2017; Shakya et al. 2019; Mengistu et al. 2019). Classification and source identification of dissolved ions in groundwater have the advantage of providing useful information for policy makers towards sustainable management of these vital resources. The quality of groundwater is influenced by physical, chemical and biological constituents and also depends on the composition of recharge water, water–rock–soil interaction in the unsaturated zone, residence time as well as geochemical and biochemical reactions that take place within the aquifer (Appelo and Postma 1999).
Literature indicates that the chemical composition of groundwater is influenced by different factors (Appelo and Postma 1999). Groundwater system in every region is characterized by a distinct chemistry owing to the chemical changes in meteoric water recharging its aquifer system (Hem 1991). According to Hounslow (1995), Andre et al. (2005), variations in the chemical quality of meteoric water are determined by factors such as the geologic constituents, composition of infiltrating rainwater, mineralogical constitution of the aquifer, duration of water–soil–rock interaction, dissolution and precipitation of mineral species and anthropogenic influence. Compositional relationships amongst dissolved species in groundwater can reveal the origin of solutes and the hydrogeochemical processes that produced the observed water chemistry (Jalali 2007; Singh et al. 2008). Assessment of the origin of the chemical constituents of groundwater may also reveal the type of chemical composition of groundwater and its subsurface environment in natural conditions (Shakya et al. 2019). The chemical composition type may represents recent freshwater infiltration which is characterized by Ca–Mg–HCO3 and Ca–HCO3 types or ion-exchange reactions characterized by Na–HCO3 type or relatively longer groundwater residence times during which interaction between the rocks and water is sustained for a longer period of time resulting in greater degree of interaction which leaches ions into groundwater/influence of local rain characterized by Na–Cl type (Shakya et al. 2019). The application of geochemical footprint in the characterization of hydrochemistry of groundwater as well as the identification of the geochemical processes that governs the water chemistry within a region is therefore crucial for the explicit understanding and knowledge broadening within the framework of sustainable management of water resources.
The effectiveness of multivariate statistical techniques in classifying and distinguishing intricate hydrochemical changes and the hidden processes that control the hydrochemical dynamics in aquifers have been extensively studied previously (Machiwal et al. 2015; Surinaidu 2016; Tay et al. 2017; Das et al. 2019). This technique has been employed with significant success as a tool in the study of groundwater geochemistry. It has been established that multivariate statistical techniques offer an important tool for the interpretation of complex water quality datasets, unravelling of hidden pollution sources and the factors (either natural or anthropogenic) responsible for these sources (Das et al. 2019). The proficiency of PCA model over the theoretical data is rooted in its ability to further expose hidden inter-variable relationships and allows the use of virtually limitless number of variables with precision. By means of raw data as variable inputs, the avoidance of errors arising from close number systems within the theoretical data is guaranteed (Tay 2015). Additionally, since each water quality parameter is treated as an independent variable, the masking effect of chemically related water quality parameter that are often grouped together is avoided (Tay 2015). The PCA model has been used to identify with precision patterns in theoretical data set of high dimension and expressing the data in such a manner that it accurately emphasizes their similarities and differences while simultaneously maintaining the characteristics of the data set that contribute most to its variance (Tay 2015). The PCA model accurately transforms observed variables within the theoretical data into a new set of uncorrelated variables and subsequently organizes them in decreasing order of importance with precision. The PCA model further shrinks the dimensionality of the data set by elucidating the association amongst large number of variables in terms of a smaller number of underlying factors without losing important information with precision (Tay 2015). Thus, the application of multivariate statistical technique assists in the interpretation of multifaceted data matrices to better understand the water quality as well as identify the possible factors that influence the water chemistry in a region.
It is worthy to note that scientific data on the chemistry of groundwater within the Asutifi-North district to allow for the proper management and development of groundwater within the district using conventional hydrogeochemical and statistical tool is either non-existent or very limited. Characterization of the water quality in this region using these tools within the framework of sustainable management is thus imperative.
The objective of this paper therefore was to assess and characterize the water quality of the Asutifi-North district using hydrogeochemical and statistical tools in order to delineate the processes that control groundwater chemistry within the district and assist the local communities who depend on these water resources as well as the managers to sustainably manage these resources.
Materials and methods
Study area
Location and physical setting
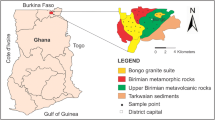
The study area lies within the Sefwi Gold Belt in the Asutifi-North district between Latitudes 6° 40′ N and 7°15′ N and Longitudes 2° 15′ W and 2° 45′ W at South-Western corridor of Brong-Ahafo Region (Fig. 1).
Topography and drainage
The topography of the study area is generally characterized by undulating landscape. It is characterized by outcrop of metamorphosed rocks in some communities such as Goaso, Konkontreso, Georgekrom. The major rivers and other waterbodies which drain the area includes River Tano and its tributaries such as Goaso, Ntotro, Akentansu, Subika, Kwamiasua, Samansua, Asundua and Subri. Other tributaries include Amoma, Kankam, Benu, Yaya and Bisi. The area lies within the middle belt of Ghana with topography ranging between 200 and 467 m above mean sea level.
Climate and vegetation
The wet semi-equatorial climatic condition prevails in and around the settlement. Annual rainfall ranges between 1400 and 1750 mm and a mean annual temperature of about 26.6 °C (Dickson and Benneh 2004). The mean monthly temperature ranges between 23 °C in August and 33 °C in March and April (Dickson and Benneh 2004). The relative humidity varies between 75 and 80% during the wet season and below 70% during the dry season (Dickson and Benneh 2004). The vegetation of the study area is predominantly moist–semi deciduous forest. Tree crops such as cocoa and citrus thrive well in this zone (Dickson and Benneh 2004).
Hydrogeological characteristics
The area is underlain mainly by Birimian Supergroup and forms part of the Paleoproterozoic supracrustal and intrusive rocks, which formed between 2195 and 2072 Ma. It exhibits a characteristic pattern of NE-trending with parallel volcanic belts and intervening sedimentary basin in the Sefwi Belt terrain (Fig. 2). The volcanic rocks in these belts are mostly intruded by synvolcanic granitoid plutons mainly granodiorite. These rocks represent tectonically stacked sequence of volcanic chain with low-grade metamorphosed, tightly to isoclinically folded sediments comprising greywackes, granitoids and granodiorites depending on relative abundance. The other major rock types in the terrain include phyllite, pyroclastic rocks and amphibolite, schist, slate, greywacke, tuff and lava (Kesse 1985). These rocks are strongly folded, foliated and jointed with associated intense weathering along fractures and other weak zones such as bedding and cleavage planes, which facilitate groundwater development. Where they crop out or are near the surface, considerable water may percolate through them. Boreholes tapping the Birimian rocks have an average yield of about 12.7 m3/h. According to existing data on boreholes in the district at the database of the Council for Scientific & Industrial Research-Water Research Institute (CSIR-WRI), borehole yields ranges between 25 and 120 lpm, depths ranges between 45 and 80 m, aquifer section ranges between 15 and 63 m depth, while static water level ranges between 1.5 and 22 m, which is generally high due to the confining nature of the aquifer. The Birimian is economically the most important geological formation in Ghana since it contains all the minerals exported from the country (Dickson and Benneh 2004).
Sampling and physico-chemical analyses
Hydrochemical
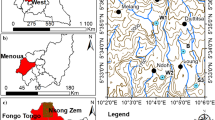
Boreholes and wells were sampled during the wet season (October 2014) and dry season (February 2015). In all, a total of 38 (No) boreholes and 6 (No) wells were investigated during the period. Figure 3 presents a map of the study area showing the sampling communities. The proposed sampling protocols described by Claasen (1982) and Barcelona et al. (1985) were rigorously followed during sampling. The samples were collected into 1000-cm3 polyethylene bottles that were previously washed and rinsed using 4–1 acid without preservation. The samples were then stored on ice in an ice-chest (Appelo and Postma 1999) and transported to the CSIR-WRI laboratories in Accra. Physical parameters such as pH, temperature and electrical conductivity were measured in the field using Hach Sens ion 156 m. In the laboratory, all samples were stored in a refrigerator at a temperature of < 4 °C until they were analysed within one week.
Suitable certified and acceptable international procedures outlined in the Standard Methods for the Examination of Water and Wastewater (APHA 2012) were used to conduct chemical analyses of the samples. The flame photometric method was used to analyse sodium (Na+) and potassium (K+); calcium (Ca2+) by EDTA titration; magnesium (Mg2+) by calculation after EDTA titration of calcium and total hardness; alkalinity and bicarbonate (HCO3−) by strong acid titration with phenolphthalein indictor; TDS by gravimetric method; nitrate-nitrogen by hydrazine reduction and spectrophotometric determination at 520 nm; sulphate by precipitation with barium chloride and spectrophotometric determination at 420 nm; Phosphate-phosphorus (PO4−P) by stannous chloride reduction and spectrophotometric determination at 690 nm; silica (SiO2) by the formation of molybdosilicate in the molybdate yellow method and spectrophotometric determination at 690 nm; chloride (Cl−) by argentometric titration and Iron (Fe2+) by Agilent 240FS atomic absorption spectrometer by direct aspiration of water samples into an air acetylene flame. As a basis for scrutinizing analytical results, an ionic balance was calculated for each chemical sample. Results with ionic balance greater that 5% were rejected in agreement with international standards (Tay et al. 2017). Charge balances (CB) were calculated based on Tay et al. (2017) as in Eq. (1):
where z is the ionic charge and M the molality, and the subscripts, a and c symbolizes anions and cations respectively.
Data analysis
The hydrochemical data were analysed using SPSS 16.0 software package. Bivariate plots used for the determination of the sources of major ions were drawn using Microsoft Excel 2013. The stability diagrams of albite and anorthite and their possible secondary weathering products were drawn with the assumption that all aluminium is preserved in the weathering products (Appelo and Postma 1999). End-member compositions were also assumed using equilibrium relationships of Tardy (1971) for standard temperature (25 °C) and pressure (1 atmosphere) which principally reflects conditions in the groundwater. Computations of constituents’ activities were done using Phreeqc for Windows Version 2.8.01 (Appelo and Postma 1999). AquaChem 4.0 was used to extract the Piper trilinear plot for the delineation of the water types. Principal component analysis (PCA) was performed using Varimax with Kaiser normalization. An eigenvalue provides a measure of the significance of the factor with, the factor having the highest eigenvalue as the most significant. Eigenvalues of 1.0 or greater are considered significant (Tay et al. 2017). Corresponding to absolute loading values of > 0.75, 0.75–0.50, 0.50–0.30, factor loadings are classified as ‘strong’, ‘moderate’ and ‘weak’ respectively (Tay et al. 2017).
Results and discussions
Hydrochemistry
Table 1 presents the statistical summary of the hydrochemical data for groundwater within the district, while Table 2 presents the classification of groundwater based on hardness values. Table 3 presents the component matrix of the main physico-chemical parameters, while Table 4 presents the total variance in the hydrochemical data.
Groundwater temperatures within the Asutifi-North district are characteristics of tropical waters and ranged 25.8–32.9 °C with mean and standard deviation value of 28.1 ± 1.9 °C. According to Stumm and Morgan (1981), these values are outside the natural background levels of 22.0–29.0 °C for waters in the tropics.
The pH values of boreholes are moderately acidic to neutral and ranged 4.4–7.5 with a mean value and standard deviation of 6.1 ± 0.7 pH units. Based on Hounslow (1995), 67.4% of groundwater are moderately acidic (pH 4–6.5). The moderately acidic characteristics of groundwater within the district may be due to the influence of aquifer materials on the chemistry of groundwater. The presence of sulphides and carbonaceous matter within the ore formations according to the geology of the catchment area suggests that natural geochemical and biochemical oxidation of these rocks may occur when in contact with oxygen gas containing water, resulting in increased hydrogen ion activity in the waters with which they are in contact. According to Tay (2015) when exposed, sulphides such as pyrites (FeS2), arsenopyrite (FeAsS) and chalcopyrite (CuFeS2) react with water and oxygen to produce sulphuric acid (H2SO4) mainly ensuing to the production of acid mine drainage. The mechanism involved in the acid mine drainage production can be explained as follows; pyrite is oxidized by oxygen in the presence of water and ferrous iron to produce Fe2+, SO42− and H+ as presented in reaction Eq. (2) (Tay 2015):
The ferrous ion (Fe2+) produced in reaction Eq. (2) is then oxidized to ferric ion (Fe3+) in the presence of Thiobacillus Ferooxidans as a catalyst in a relatively slow reaction as depicted by Eq. (3):
According to Tay (2015), the reaction Eq. (3) is the major rate-determining step of acid mine drainage formation. Any further development of the acid drainage process is dependent on the pH of the water of interest. If the pH > 3 as is the case of groundwater within the Asutifi-North district where the pH range was 4.4–7.5; then, Fe(OH)3 is principally precipitated as presented in reaction Eq. (4):
Thus, acid mine drainage reactions in groundwater within the Asutifi-North district principally produces Fe(OH)3. It is noteworthy to indicate that the oxidation of arsenopyrite (FeAsS) and other sulphide minerals by oxygen through proton production undergoes analogous reactions (Tay 2015). Additionally, the moderately acidic characteristics coupled with the low SO42− concentration and low conductivity of most of the shallow groundwater suggests that acidity could result from the production of carbonic acid from atmospheric CO2 during rainfall or through the solution of soil-generated CO2 and dissolved organic acids such as fulvic and humic acids (Langmuir 1997).
Groundwater within the district was characteristically low to highly mineralized as the electrical conductivity ranged 26.0–1377.0 μS/cm, with a mean value of 445.4 ± 336.0 μS/cm. The electrical conductivity values shows that 52.2% of groundwater within the district can be classified as fresh waters (EC < 500 μS/cm) according to the classification by Hounslow (1995). Total dissolved solids (TDS) ranged 14.3–757 mg/L with mean and standard deviation of 224.9 (± 183.5) mg/L. According to Hounslow (1995), groundwater within the district perhaps undergoes silicate weathering as 95.6% of groundwater had TDS values < 500 mg/L. The high standard deviation in TDS and EC reflects the spatial variability in ionic concentrations in groundwater within the district. The large spatial differences in TDS values are perhaps due to lithological variations in the weathering of anorthite and albite present in the rocks and/or local impact of anthropogenic activities within the district. However, groundwater within the district is generally soft to moderately hard. Hardness values ranged 8.8–492.0 mg/L with a mean value and standard deviation of 159.8 (± 123.8) mg/L. Total hardness is due to Ca2+ and Mg2+ in water. The high standard deviation in total hardness may be due to spatial variability in Ca2+ and Mg2+ concentrations in groundwater within the district. Todd (1980) classified water hardness into four categories as soft, moderately hard, hard and very hard. Table 2 presents the different classifications of water hardness. Out of the 46 data points, 34.8% are soft, 13.0% are moderately hard, 41.3% are hard and 10.9% are very hard. Based on this classification, only 47.8% of the groundwater in the district can be said to be potable for drinking and domestic purposes.
Groundwater alkalinity within the district ranged 8.0–336.0 mg/L with a mean and standard deviation of 118.1 ± 88.8 mg/L. The chemical constituents generally have low concentrations (Table 1) and are within the WHO (2004) Guideline values. The boxplot of the major ion concentrations in groundwater within the district is presented in Fig. 4. The error bars in Fig. 4 depicts the spatial variability in ionic concentrations in groundwater within the district. From Fig. 4, HCO3− is the dominant anion in groundwater within the district. Tay et al. 2017 showed that for groundwaters with HCO3− as dominant anions, groundwater composition takes place at outcrops. Compared with other cations, the concentration of Ca2+ is relatively high. The relative abundance of cations and anions in groundwater within the district is in the order of: Ca2+ > Na+ > Mg2+ > K+ and HCO3− > Cl− > SO4 2− respectively.
NO3−N in groundwater within the district ranged 0.005–3.77 mg/L, with a mean and standard deviation value of 0.43 ± 0.2. NH4−N in groundwater within the district ranged 0.04–0.62 mg/L, with a mean and standard deviation value of 0.3 ± 0.2. Nitrate is a significant pollutant found in the environment which is a derivative of atmospheric precipitation, human an animal excreta, agricultural fertilizers, biological fixation and nitrification of organic N and NH4 (Tiwari 2014). NO3−N concentrations in groundwater within the district is within the WHO guideline value of 10 mg/l for drinking water. This could be due to the low levels of anthrogenic activities and local precipitation within the district. PO4−P in groundwater within the district ranged 0.02–0.7 mg/L, with a mean and standard deviation value of 0.19 ± 0.15. SiO2 in groundwater within the district ranged 0.29–1.7 mg/L, with a mean and standard deviation value of 0.9 ± 0.4.
Gibbs (1970) has shown that generally, the chemistry of water is controlled by the rate of evaporation, chemical compositions of the rocks (rock weathering) and chemical composition of rainwater (precipitation). Figure 5 presents the scatter plot of TDS (mg/L) vs. Na+/(Na+ + Ca2+) (meq/L) based on Gibbs (1970) showing the mechanisms controlling groundwater chemistry within the Asutifi-North district. Figure 5 shows that rock dominance weathering and precipitation dominance contribute to the hydrogeochemical processes responsible for the chemical evolution of groundwater within the district. According to Hounslow (1995), in environments where the pH is low, it is usually the result of weak acids such as carbonic acid resulting from precipitation or dissolved organic acids derived from decaying organic matter. Consistent with Hounslow (1995), some of the groundwater have moderately low pHs. Thus, the chemistry of their waters may be controlled by precipitation (Fig. 5). Das et al. (2019), in their assessment of groundwater in the Birbhum District West Bengal, India, using Gibbs diagram, showed that the mechanism controlling groundwater in the district was the combination of rock weathering and resultant mixing of minerals. Hussien and Faiyad (2016) employed the Gibbs diagram to determine the mechanism that controls the groundwater of UmErdhuma–Tayarat aquifer situated in a region where dry and semiarid climatic conditions are dominant. The scientists reported that evaporation–crystalization processes significantly control the major ion chemistry of groundwater perhaps due to dry and semiarid climatic conditions. A study on characterization of recharge mechanism and sources of groundwater salinization in Ras Jbel Coastal Aquifer, Northeast Tunisia, conducted by Abidi et.al. (2017) also employed the Gibbs diagram to investigate the mechanism controlling groundwater in the region. The study showed that there was a combination of rock weathering (weathering of carbonate minerals) as well as the evaporation–crystalization dominances significantly controlling the major ion chemistry of the groundwaters in the Ras Jbel Coastal Aquifer.
Sources of major ions
According to Hounslow (1995), for groundwater with major ion ratios of Na+/(Na+ + Cl−) > 0.5, the origin of sodium is either albite or ion-exchange. Results shows that 66.6% of the groundwater had Na+/(Na+ + Cl−) ratios > 0.5, suggesting either albite weathering or ion-exchange reactions as sources of sodium in groundwater within the district. According to Hounslow (1995), in a plot of Na+ vs. Cl−, groundwater plotting close to the 1:1 line suggest sodium source from sea aerosol while groundwater plotting above the 1:1 line suggests ion-exchange and groundwater plotting below the 1:1 line suggests reverse ion-exchange. Results from this study shows that majority of groundwater plotted either close to or above the 1:1 line (Fig. 6), suggesting sodium source from either sea aerosol spray or sodium source other than halite; probably, albite (plagioclase) weathering or ion-exchange is partly responsible for Na+ in groundwater within the district. However, the district is not close to the coast; therefore, sodium source from sea aerosol spray is also unlikely. Thus, albite (plagioclase) weathering or ion-exchange may be partly responsible for Na+ in groundwater within the Asutifi-North district. This result is consistent with Narany et al. (2014) who conducted hydrogeochemical assessment of groundwater within the Amol-Babol Plain in Iran and reported that the groundwater principally showed a Na + /Cl− ratios ≥ 1 and therefore represents silicate weathering processes in groundwater within the Amol-Babol plain. Sources of Cl− in groundwater include both natural and anthropogenic. Atmospheric precipitation, weathering of halite and dissolution of salt deposits are perhaps the major lithogenic or natural sources of Cl− in groundwater within the district. Anthropogenic sources of Cl− in groundwater within the district may include animal wastes, chemical fertilizers and leachates from waste dumps.
Datta et al. (1996), Rajmohan and Elango (2004) and Sandow (2009) showed in their studies that waters plotting above the equiline (1:1) in a plot of (HCO3− + SO42−) vs. (Ca2+ + Mg2+), shows a dominance of carbonate weathering while waters plotting below the 1:1 line depicts a dominance of silicate weathering. Figure 7 shows that the groundwater plot above and below the 1:1 line suggesting both carbonate and silicate weathering dominance in groundwater within the district. Additionally, according to McLean and Jankowski (2000), in a plot of (HCO3− + SO42−) vs. (Ca2+ + Mg2+), a 1:1 relationship would indicate gypsum, anhydrite, calcite and dolomite dissolution as the predominant processes controlling solution composition while, groundwater falling below the 1:1 line depicts ion-exchange, in which case Ca2+ + Mg2+are depleted with respect to HCO3− + SO42−. As shown in Fig. 7, majority of the waters plot close to and on the 1:1 line, while, a few plot above and below the1:1 line, suggest gypsum, anhydrite, calcite and dolomite dissolution as well as ion-exchange reactions controlling solution composition in groundwater within the district. Approximately, 88.8% of groundwater also shows that, the Ca2+/(Ca2+ + SO42−) equivalent ratios were greater 0.5, suggesting calcium source other than gypsum-carbonates, calcite/dolomite or silicates. Additionally, 77.7% of groundwater had HCO3−/SiO2 ratios > 10, suggesting carbonate weathering reactions. Thus, calcite/dolomite, carbonates or silicate dissolution may have contributed significantly to Ca2+ and Mg2+concentrations in groundwater within the district. This result is consistent with Narany et al. (2014) who reported that majority (73.6%) of groundwater within the Amol-Babol Plain in Iran plotted close to the 1:1 line in a plot of (HCO3− + SO42−) vs. (Ca2+ + Mg2+), and therefore, an excess of Ca2+ and Mg2+ over HCO3− + SO42− and attributed it to the dissolution of calcite, dolomite and gypsum. Mohammed and Zineb (2015) also investigated the hydrogeochemical processes in groundwater in Souf Valley of Low Septentrional Sahara in Algeria and reported that carbonate and silicate weathering are the major hydrogeochemical processes that governs the groundwater chemistry within the Souf Valley.
Generally, the dissolution of carbonate minerals such as calcites and dolomites may be represented by Eqs. (5) and (6), respectively (Al-Amry 2008):
According to Al-Amry (2008), the molar proportions of (Ca2+ + Mg2+): (HCO3−) should be linear with a slope of 1, if dolomite dissolution takes place in groundwater. The scatter plot of (Ca2+ + Mg2+) vs. (HCO3−) in groundwater within the district is presented in Fig. 8. From Fig. 8, the slope is 0.6983 which is close to the expected (i.e. a slope of 1). There suggests a general excess of (Ca2+ + Mg2+) over (HCO3−) in groundwater within the district. This is supported by the hydrochemical data as 77.5% of groundwater had ratios of (Ca2+ + Mg2+)/HCO3− > 1. This does not support the general trend predicted by the dolomite dissolution model (Ca2+ + Mg2+)/HCO3 = 1, and reflects additional sources of Ca2+ and Mg2+ in groundwater within the district. This suggests the presence of other carbonate minerals within the subsurface.
The incongruent dissolution of albite to produce the secondary mineral products; kaolinite, montmorillonite and/or gibbsite according to Kortatsi (2004) can be presented in Eqs. (7), (8) and (9), respectively:
The H4SiO4/Na+ molar ratios for the dissolution of albite to kaolinite, montmorillonite and gibbsite as presented in Eqs. (7), (8) and (9) are theoretically 2.0, 0.5 and 3.0, respectively (Kortatsi 2004). The calculated H4SiO4/Na+ molar ratios for groundwater within the district ranged 0.4–2.7, with a mean value of 1.6. Clearly, the result suggests that weathering processes in which albite is dissolved into kaolinite and Na-montmorillonite with molar ratios of 2.0 and 0.5, respectively, are taking place in groundwater within the district. Mineral stability diagrams have been widely employed to explicitly explain weathering processes involving silicate minerals in order to establish the most stable silicate mineral phases in the hydrochemical assessment of groundwater (Kashiwagi et al. 2006). The stability of albite and its secondary weathering products gibbsite, kaolinite and Na-montmorillonite with respect to groundwater within the district is presented in Fig. 9. All (100%) groundwater plotted in the kaolinite and Na-montmorillonite fields on the stability diagram of albite and its secondary weathering products (Fig. 9). The calculated molar ratios of kaolinite and montmorillonite are consistent with the data plot in Fig. 9. As shown in Table 1, SiO2 concentrations in groundwater within the district ranged between 0.29 and 1.7 mg/L, with a mean standard deviation value of 0.9 ± 0.4. This range falls within the general range of SiO2 concentrations found in natural waters probably reflecting the low solubility of common silicate minerals (Yidana et al. 2012) such as albite and anorthite present in the geology of the district. Additionally, the pH of groundwater typically have minimum effect on the solubility of SiO2, except at pH > 9.0 where the solubility of silicates increases speedily (Yidana et al. 2012). From Table 1, the pH of groundwater within the district is less than 9 (i.e. ranged from 4.4 to 7.5 pH units, and a mean value and standard deviation of 6.1 ± 0.7). Thus, according to Yidana et al. (2012), groundwater pH within the Asutifi-North district cannot provide the needed environments for increase in the rate of solubility of silicates. This further explains the low solubility of SiO2 in groundwater within the district.
To further ascertain the source of Na+ in groundwater, Jankowski et al. (1998) hypothesized that waters undergoing ion-exchange involving Na+ and Ca2+ along the groundwater flow path would normally plot along a line whose slope is − 1 in a plot of Na–Cl (meq L−I) vs. Ca + Mg − HCO3 + SO4 (meq L−I) while, waters plotting close to the zero value on the x-axis are not governed by ion-exchange. The plot of Na–Cl (meq L−I) vs. Ca + Mg − HCO3 + SO4 (meq L−I) for groundwater within the district is presented in Fig. 10. This study shows that the waters plot along a line whose slope is − 0.87 (Fig. 10). This slope is closer to − 1 than the zero value on the x-axis. This result is also consistent with the plot in Fig. 6 which showed that albite (plagioclase) weathering or ion-exchange may be partly responsible for Na+ in groundwater within the district. Cation exchange reactions are therefore one of the dominant processes through which Na+ enters the groundwater system within the district. Thus, cation exchange reactions and albite (plagioclase) dissolution are perhaps the dominant processes through which Na+ enters the groundwater within the Asutifi-North district. This result is consistent with Sajil Kumar and James (2016) who investigated the hydrogeochemical processes in groundwater in the Coimbatore District, Tamil Nadu, India, and reported a slope of − 0.938 in a plot of Na–Cl vs. Ca + Mg − HCO3 + SO4 due to a correlation between Ca, Mg and Na through ion-exchange processes.
A further source-rock deduction studies using major ion ratios shows that 62.9 % of the groundwater within the district shows 0.2 < (Na++K+ − Cl−)/(Na++K+ − Cl−+Ca2+) < 0.8, suggesting that anorthite (plagioclase) (Na0.62Ca0.38Al1.38Si2.62O8) weathering is possible.
The incongruent dissolution of anorthite (plagioclase) with carbon dioxide charged water to produce Na+ and Ca2+ according to Kortatsi (2004) can be represented as in Eq. (10):
Notwithstanding, the weathering of anorthite (plagioclase) in Eq. (10) hypothetically has molar ratios for H4SiO4/HCO3−and H4SiO4/Na+ as 0.9 and 2.0 respectively (Kortatsi 2004). Meanwhile, the computed molar ratios for H4SiO4/HCO3−and H4SiO4/Na+ for groundwater within the Asutifi-North district ranged 0.8–2.3 with a mean value of 1.7. The hydrochemical data therefore suggests that anorthite weathering to produce kaolinite and Ca-montmorillonite markedly explain the excess of Na+ over Cl− as well as the sources of Ca2+, H4SiO4 and HCO3−in groundwater within the district. Thus, anorthite (plagioclase) weathering in groundwater within the district contributes to the concentration of Na+, Ca2+, H4SiO4 and HCO3−. This result is consistent with the data plot on the stability diagram of anorthite (plagioclase) and its possible secondary weathering products (Fig. 11) where all data plot in the kaolinite and Ca-montmorillonite due to Ca2+ and SiO2 contents in the groundwater. Literature indicates that in groundwaters hosted by silicate minerals, interaction between the rocks and groundwater results in the leaching of Ca2+ and H4SiO4 ions into the groundwater system (Yidana et al. 2012). Such interactions result in different products depending on the residence time, the climate and the flow regime (Yidana et al. 2012). Thus kaolinite and Ca-montmorillonite are the most likely secondary minerals from the dissolution of anorthite (plagioclase) in groundwater within the Asutifi-North district.
Schoeller (1965, 1977) proposed two chloro-alkaline indices—CAI1 and CAI2 to clearly explain ion-exchange reactions between groundwater and its host environment during residence time. Computation of CAI1 and CAI2 indices for groundwater within the district shows that CAI1 indices ranged − 1.9 to 0.8 with a mean value of − 0.4, while CAI2 indices ranged − 0.5 to 0.8 with a mean value of 0.0. Majority of the computed CAI1 and CAI2 indices are negative suggesting an exchange of Mg2+ and Ca2+ from groundwater within the district with Na+ and K+ in the rocks. Thus, the computed chloro-alkaline indices is consistent with the plot of Na–Cl(meq L−I) vs. Ca + Mg – HCO3 + SO4 (meq L−I) and therefore suggest that cation-exchange reactions are primarily taking place in groundwater within the Asutifi-North district in which case, Ca2+ and Mg2+ are being exchanged with Na+ and K+ in the rocks.
The common sources of HCO3− in groundwater within the Asutifi-North district include weathering of silicate and/or alluminosilicate minerals (albite and anorthite), calcite and dolomite. The relatively high proportion of HCO3− compared to other anions indicates the weathering of primary silicate minerals dominated by alkaline earths (Tiwari, 2014). SO42− concentrations in natural water ranges between 2 and 80 mg/L with higher concentrations attributable to the weathering of sulphide minerals or anthropogenic sources such as agricultural effluents (Tiwari 2014). SO42− concentrations in groundwater within the district ranged 0.3–117.0 mg/L, with a mean value of 26.2, and this may possibly be due to chemical products such as ammonium sulphate fertilizers via agricultural activities and the weathering of sulphide minerals. The oxidation of sulphides (pyrites and arsenopyrites) via acid mine drainage is also generally expected to be a significant source of SO42− in groundwater (Tay 2015). The oxidation reactions is as presented in Eqs. (2), (3) and (4). Traditionally, these reactions produce high SO42− concentrations, low pH and Fe2+/SO42− molar ratios of 0.5 (for the stoichiometry of pyrite oxidation) and 1.0 (for the stoichiometry of arsenopyrite oxidation) (Tay 2015). However, the generally very low concentration of SO42− which ranged 0.003–1.22 mmol/L, in addition to the fact that, no groundwater satisfied the Fe2+/ SO42− molar ratio of 0.5 and 1.0 for the stoichiometry of pyrite and arsenopyrite oxidation, respectively, suggests that pyrite and arsenopyrite oxidation processes are not exclusively responsible for the concentration of SO42− in groundwater within the district.
Hydrochemical facies
Figure 12 presents the Piper (1944) trilinear plot of the hydrochemical data for the Asutifi-North district. From Fig. 12, majority (68.1%) of the data points clustered towards the Ca–Mg–HCO3 dominant section (II) indicating water type characterized by temporary hardness. The Ca–Mg–HCO3 water type denotes vigorous recharge and/or association of groundwater with relatively soluble silicate minerals within the aquifer. Surface water charged with atmospheric carbon dioxide and biogenically carbon dioxide charged water infiltrating the subsurface and reacting with silicates/aluminosilicates such as plagioclase present in the geology of the district. Subsequently, Ca2+ and Mg2+ are released into the groundwater within the district. Plummer et al. (1990), Edmunds and Smedley (2000), Adams et al. (2001) have all reported from geochemical groundwater evolution studies from several parts of the world that the Ca–Mg–HCO3 water type are commonly regarded as recharge area waters at their early phase of geochemical evolution and are rapidly circulating groundwater which have not undergone pronounced water–rock interaction. Some (13.6%) of the groundwater clustered towards the Na–Cl dominant section (III). It has been indicated earlier that the district is not close to the coast, and therefore, the source Na–Cl water type in groundwater within the district is not from sea aerosol spray but may be due to the influence of local rain and depicts the significance of local recharge conditions within the district. Some (11.4%) of the groundwater also clustered towards the Ca–Mg–Cl dominant section (V) where no pair dominates. The sources of the Ca–Mg–Cl water type may be due to the mixing of recharge area waters at their early phase of geochemical evolution with local rain and/ or ion-exchange between Ca–Mg–HCO3 and Na–Cl water types. Thus, the Ca–Mg–Cl water type is characteristic of mixed waters where neither cation nor anion dominates and are reminiscent of permanent hard waters. A few (6.8%) of the groundwater also clustered towards the Ca–Mg–SO4–Cl dominant section (I). The Ca–Mg–SO4–Cl water type is reminiscent of permanent hard waters. 2.3% of the groundwater also clustered towards the Na–K–HCO3 dominant section (VI) where no pair dominates. The Na–K–HCO3 water type is characteristic of mixed waters where neither cation nor anion dominates. Shakya et al. (2019), employed the Piper (1944) trilinear plot in the investigation of deep groundwater recharge processes in an intermountain basin in the Kathmandu Valley, Nepal. The study reported that the Na–K–HCO3, Ca–Mg–HCO3, Na–K–HCO3 and Ca–Mg–HCO3 type waters were mainly found in the Kathmandu Valley. Na–K–HCO3, Ca–Mg–HCO3 type waters depicted the influence of geological settings while, Na–K–HCO3 type water was due to groundwater interaction with gneiss, and Ca–Mg–HCO3 type water was due to the groundwater interaction limestone bedrock.
Principal component analysis (PCA)
PCA led to the extraction of three (3) main principal components which identifies the factors that influence each principal components for the physico-chemical parameters. The three principal components have accounted for approximately 73.1% of the total variance in the hydrochemical data and have eigenvalues > 1. According to Jolliffe et al. (2016), a limit of cumulative 70% variation is common to retain the PCs for any analysis. A total variance of 73.1% was therefore statistically significant to retain the PCs for this study.
Component 1 explains nearly 42.7% of the total variance (Table 4) and has strong positive loadings (> 0.75) for EC, TDS, Total hardness, Ca2+, Na+, K+, and a moderate positive loadings (0.75–0.50) for pH, Mg 2+, K+, HCO3−, Cl− and SO42− (Table 3) suggesting that the major ions significantly contribute to the total dissolved solids of the groundwater and can be accounted for by major geochemical processes within the aquifer. Component 2 explains approximately 21.6% of the total variance (Table 4) and has moderate positive loadings for Cl− and pH and weak positive loadings for SiO2, NO3-N, NH4-N and HCO3−. This reflects a common source, clearly silicate/aluminosilicate weathering by carbon dioxide charged water. The moderate to weak positive loadings for silica, HCO3− and pH in the groundwater suggests sorption of silica by clay minerals and signifies the influence of carbonate mineral weathering on the hydrochemistry of groundwater within the district (Yidana et al. 2012). It is most probable that dissolved silica in the groundwater within the district originates from the chemical breakdown of silicates during weathering processes. Component 2 also has a weak positive loading for NO3−N and a weak negative loading for NH4−N reflecting a common source of anthropogenic origin (possibly pollution from inorganic fertilizers such as nitrates and ammonia).
Component 3 explains approximately 8.8% of the total variance (Table 4) and has strong positive loading for PO4-P. Component 3 also shows a possible pollution from inorganic fertilizers containing phosphates. The economic activity within the district includes farming where, foodstuffs such as yam, plantain, banana, vegetables, fruits and cash crops such as cocoa, oil palm and coffee are grown. Land degradation as a result of poor farming practices where indiscriminate use of nitrogen and phosphorus-based fertilizers are widespread. These are some of the human induced activities which are most likely to have anthropogenic impact on the water resources within the district.
Thus, from the PCA, it can be inferred that Component 1 delineates the main natural processes(water–soil–rock interactions) through which groundwater within the district acquires its chemical characteristics, Component 2 delineates the incongruent dissolution of silicate/aluminosilicates, as well as human-induced pollution via agriculture, while Component 3 delineates the prevalence of pollution principally from agricultural inputs. This study is consistent with Rao et al. (2007), who employed PCA in groundwater assessment in an urban environment of Andhra Pradesh, India. The scientists reported that three (3) principal components accounted for 77.20% of the total variance with eigenvalues > 1 in the hydrochemical data. Component 1 delineated salinity processes, while Component 2 delineated alkalinity processes and Component 3 delineated the prevalence of pollution principally from agricultural inputs.
The component plot in rotated space is presented in Fig. 13. Figure 13 shows consistency with the PCA results where the major ions involved in mineralization of the groundwater have been extracted together in Component 1. SiO2, HCO3− and pH are extracted together in Component 2, and PO4-P has been extracted in Component 3.
Conclusion
The hydrogeochemical evaluation shows that the pH values of groundwater within the district are moderately acidic to neutral. Approximately 67% of groundwater acidity was due principally to the influence of aquifer materials on the chemistry of groundwater. The low pH of groundwater within the district significantly influences silicate mineral weathering which is an active process in the groundwater flow system. The study further shows that acid mine drainage processes principally produces Fe(OH)3 in groundwater within the district. The major ion concentrations are within the WHO (2004) Guideline values for drinking water. The relative abundance of cations and anions in groundwater within the district was in the order of: Ca2+ > Na+ > Mg2+ > K+ and HCO3− > Cl− > SO42−, respectively. The study further shows that plagioclase (albite and anorthite), carbonates (calcite and dolomite) dissolutions as well as ion-exchange reactions may have contributed significantly to major ion concentrations in groundwater within the district. Hydrochemical facies using Piper trilinear diagrams shows principally signatures of association of groundwater with relatively soluble silicate minerals (albite and anorthite) within the aquifer and the influence of local rain through vigorous recharge by local precipitation within the district. Other minor signatures from ion-exchange and active mixing of local flow have also been delineated. PCA has resulted in the extraction of three (3) main principal components accounting for approximately 73.1% of the total variance with eigenvalues > 1 in the hydrochemical data. The three (3) main principal components represents the main natural processes through which groundwater within the district acquires its chemical characteristics, the incongruent dissolution of silicate/aluminosilicates minerals to produce kaolinite and Na–/Ca–montmorillonite as the secondary minerals from the dissolution of plagioclase and the prevalence of pollution principally from the application of inorganic fertilizers such as nitrates and ammonia via agricultural activities within the district.
References
Abidi JH, Farhat B, Mammou AB, Oueslati N (2017) Characterization of recharge mechanisms and sources of groundwater salinization in Ras Jbel coastal aquifer (Northeast Tunisia) using hydrogeochemical tools, environmental isotopes, GIS, and statistics. J Chem (Hindawi) 2017:1–20. https://doi.org/10.1155/2017/8610894
Adams S, Tredou G, Harris C, Titus R, Pieterson K (2001) Hydrochemicalcharacteristics of aquifers near Sutherland in the Western Karoo, South Africa. J Hydrol 24:91–103
Al-Amry AS (2008) Hydrogeochemisry and groundwater quality assessment in an arid region: a case study from Al Salameh Area, Shabwah, Yemen. In: The 3rd international conference on water resources and arid environments and the 1st Arab water forum
Andre L, Franceschi M, Pouchan P, Atteia O (2005) Using geochemical data and modeling to enhance the understanding of groundwater flow in a regional deep aquifer, Aquitaine Basin, south-west of France. J Hydrol 305:40–62
APHA-AWWA-WEF (2012) Standard methods for the examination of water and wastewater, 22nd edn. American Public Health Association, New York
Appelo CAJ, Postma D (1999) Geochemistry, groundwater and pollution, 2nd edn. A.A. Balkema, Rotterdam, p 649
Barcelona M, Gibb JP, Helfrich JA, Garske EE (1985) Practical guide for groundwater sampling. Illinois State Water Survey ISWS Contract Report 374
Claasen HC (1982) Guidelines and techniques for obtaining water samples that accurately represent the quality of an aquifer. US Geol Surv Open File Rep. 82(1024):49
Das N, Mondal P, Ghosh R (2019) Sutradhar S (2019) Groundwater quality assessment using multivariate statistical technique and hydro-chemical facies in Birbhum District, West Bengal. India SN Appl Sci 1:825. https://doi.org/10.1007/s42452-019-0841-5
Datta PS, Tyagi SK (1996) Major ion chemistry of groundwater of Delhi area: chemical weathering processes and groundwater flow regime. J Geol Soc India 47:179–188
Dickson KB, Benneh G (2004) Anew geography of Ghana, revised edn, Fifth impression, pp 36–38
Edmunds WM, Smedley P (2000) Residence time indicators in groundwater: the East Midlands Triassic sandstone aquifer. J Appl Geochem 15:737–752
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170:1088–1090
Hem (1991) Study and interpretation of the chemical characteristics of natural water. US Geochemical Survey Water Supply Paper 2254, Scientific Publishers, India
Hounslow AW (1995) Water quality data analysis and interpretation. Lewis Publishers, Boca Raton
Hussien BM, Faiyad AS (2016) Modeling the hydrogeochemical processes and source of ions in the groundwater of aquifers within Kasra-Nukhaib region, West Iraq. Int J Geosci 7:1156–1181
Jalali M (2007) Assessment of the chemical components of Famenin groundwater, western Iran. Environ Geochem Health 29:357–374
Jankowski J, Acworth RI, Shekarforoush S (1998) Reverse ion-exchange in deeply weathered porphyritic dacite fractured aquifer system, Yass, New South Wales, Australia. In: Arehart GB, Hulston JR (eds) proceedings of 9th international symposium water–rock interaction. Taupo, New Zealand, 30th March–April 1998, Balkema, Rotterdam, pp 243–346
Jolliffe IT, Cadima J (2016) Principal component analysis: a review and recent developments. Philos Trans R Soc A Math Phys Eng Sci 374(2065):20150202
Kashiwagi H, Shikazano N, Ogawa Y, Higuchi Y, Takahashi M, Tanaka Y (2006) Mineralogical and biological influences on groundwater chemistry of the Boso Peninsula, Chiba Central Japan. Geochem J 40:345–361
Kesse GO (1985) The mineral and rock resources of Ghana. AA Balkema, Boston
Kortatsi BK (2004) Hydrochemistry of groundwater in the mining area of Tarkwa-Prestea. Ghana. Unpublished PhD Thesis. University of Ghana, Legon-Accra
Langmuir D (1997) Aqueous environmental geochemistry. Prentice-Hall Inc, ., p 601
Machiwal D, Jha MK (2015) Identifying sources of groundwater contamination in a hard-rock aquifer system using multivariate statistical analyses and GIS-based geostatistical modeling techniques. J Hydrol Reg Stud 4:80–110
McLean W, Jankowski J (2000) Groundwater quality and sustainability in an alluvial aquifer, Australia. In: Sililo et al (eds) Proceedings of XXX IAH congress on groundwater: past achievements and future challenges. Cape Town South Africa 26th November-1st December 2000. AA Balkema, Rotterdam, Brookfield
Mengistu HA, Demlie MB, Abiye TA, Xu A, Kanyerere T (2019) Conceptual hydrogeological and numerical groundwater flow modelling around the Moab Khutsong deep gold mine South Africa. Groundw Sustain Dev 9:100266
Mohamed C, Zineb A (2015) Geochemistry and hydrogeochemical process of the groundwater in the Souf valley of low septentrional Sahara, Algeria. Afr J Environ Sci Technol 3:261–273
Narany TS, Ramli MF, Aris AZ, Sulaiman WNA, Juahir H, Fakharian K (2014) identification of the hydrogeochemical processes in groundwater using classic integrated geochemical methods and geostatistical techniques, in Amol-Babol Plain. Iran Sci World J (Hindawi) 2014:1–15. https://doi.org/10.1155/2014/419058
World Health Organization (2012) Guidelines for drinking-water quality, 4th edn. World Health Organization, Geneva
Piper AM (1944) A graphic procedure in the geochemical interpretation of water analyses. Am Geophys Union Trans 25:914–923
Plummer L, Busby J, Lee R, Hanshaw B (1990) Geochemical modelling of the Madison aquifer in parts of Montana, Wyoming, and South Dakota. Water Resour Res 26:1981–2014
Rajmohan N, Elango L (2004) Identification and evaluation of hydrogeochemical processes in the groundwater environment in an area of the Palar and Cheyyar river basins, Southern India. Environ Geol 46:47–61
Rao SN, Rao JP, Subrahmanyam A (2007) Principal component analysis in groundwater quality in a developing urban area of Andhra Pradesh. J Geol Soc India 69:959–969
Sajil Kumar JA, James EJ (2016) Identification of hydrogoechemical processes in the Coimbatore districts, Tamil Nadu. India Hydrol Sci J 61(4):719–731
Sandow MY (2009) The hydrochemical framework of surface water basins in southern Ghana. Environ Geol 57:789–796
Schoeller H (1965) Qualitative evaluation of groundwater resource. In: Methods and techniques of groundwater investigation and development. UNESCO, pp 54–83
Schoeller H. (1977) Geochemistry of groundwater. In: Groundwater Studies- An International Guide for Research and Practice. UNESCO, Paris, Ch.15, 1–18.
Shakya BM, Nakamura T, Shrestha SD, Nishida K (2019) Identifying the deep groundwater recharge processes in an intermountain basin using the hydrogeochemical and water isotope characteristics. Hydrol Res 50(5):1216–1229
Singh AK, Mondal GC, Kumar S, Singh TB, Tewary BK, Sinha A (2008) Major ion chemistry, weathering processes and water quality assessment in upper catchment of Damodar river basin. India Environ Geol 54:745–758
Stumm W, Morgan JJ (1981) Aquatic chemistry. Willey, New York, p 780
Surinaidu L (2016) Role of hydrogeochemical process in increasing groundwater salinity in the central Godavari delta. Hydrol Res 47(2):373–389
Tardy Y (1971) Characterization of the principal weathering types by the geochemistry of waters from some European and African crystalline massifs. ChemGeol 7:253–271
Tay C (2015) Hydrogeochemical processes influencing groundwater quality within the Lower Pra Basin, Ghana. Published PhD Thesis submitted to the Institute for Environment and Sanitation Studies, University of Ghana, Legon. Available at http://ugspace.ug.edu.gh
Tay C, Hayford E, Hodgson IO (2017) Application of multivariate statistical techniques for hydrogeochemical assessment of groundwater quality within the lower pra basin, Ghana. Appl Water Sci 7:1131–1150. https://doi.org/10.1007/s13201-017-0540-6
Tiwari AK, Singh AK (2014) Hydrogeochemkical investigation of groundwater quality assessment of Pratapgarh district, Uttar Pradesh. J Geol Soc India 83:329–343
Todd DK (1980) Groundwater hydrology. Wiley, New York
World Health Organization (WHO) (2004) Guidelines for drinking water quality. Revision of the 1993 guidelines. Final Task Group Meeting. Geneva, 2003
Yidana SM, Banoeng-Yakubo B, Sakyi PA (2012) Identifying key processes in the hydrochemistry of a basin through the combined use of factor and regression models. J Earth Syst Sci 121(2):491–507
Acknowledgments
The author is grateful to the Government of Ghana through the Council for Scientific and Industrial Research-Water Research Institute for its assistance for this study. I am also grateful to Groundwater Division of the CSIR-WRI for the maps of the study area.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tay, C.K. Hydrogeochemical framework of groundwater within the Asutifi-North District of the Brong-Ahafo Region, Ghana. Appl Water Sci 11, 72 (2021). https://doi.org/10.1007/s13201-021-01398-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-021-01398-1