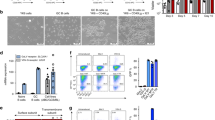
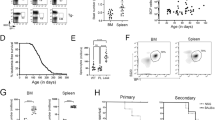
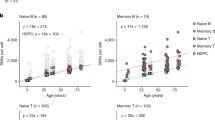
Abstract
Next-generation sequencing has transformed our knowledge of the genetics of lymphoid malignancies. However, limited experimental systems are available to model the functional effects of these genetic changes and their implications for therapy. The majority of mature B-cell malignancies arise from the germinal center (GC) stage of B-cell differentiation. Here we describe a detailed protocol for the purification and ex vivo expansion of primary, nonmalignant human GC B cells. We present methodology for the high-efficiency transduction of these cells to enable combinatorial expression of putative oncogenes. We also describe alternative approaches for CRISPR–Cas9-mediated deletion of putative tumor suppressors. Mimicking genetic changes commonly found in lymphoid malignancies leads to immortalized growth in vitro, while engraftment into immunodeficient mice generates genetically customized, synthetic models of human lymphoma. The protocol is simple and inexpensive and can be implemented in any laboratory with access to standard cell culture and animal facilities. It can be easily scaled up to enable high-throughput screening and thus provides a versatile platform for the functional interrogation of lymphoma genomic data.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All raw data underlying the figures are provided as source data files. Other examples of data can be made available upon reasonable request. Source data are provided with this paper.
References
Basso, K. & Dalla-Favera, R. Germinal centres and B cell lymphomagenesis. Nat. Rev. Immunol. 15, 172–184 (2015).
Basso, K. & Dalla-Favera, R. BCL6: master regulator of the germinal center reaction and key oncogene in B cell lymphomagenesis. Adv. Immunol. 105, 193–210 (2010).
Shaffer, A. L. 3rd, Young, R. M. & Staudt, L. M. Pathogenesis of human B cell lymphomas. Ann. Rev. Immunol. 30, 565–610 (2012).
Morin, R. D. et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature 476, 298–303 (2011).
Pasqualucci, L. et al. Analysis of the coding genome of diffuse large B-cell lymphoma. Nat. Genet. 43, 830–837 (2011).
Lohr, J. G. et al. Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma (DLBCL) by whole-exome sequencing. Proc. Natl Acad. Sci. USA 109, 3879–3884 (2012).
Zhang, J. et al. Genetic heterogeneity of diffuse large B-cell lymphoma. Proc. Natl Acad. Sci, USA 110, 1398–1403 (2013).
Reddy, A. et al. Genetic and functional drivers of diffuse large B Cell Lymphoma. Cell 171, 481–494 e415 (2017).
Schmitz, R. et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N. Engl. J. Med. 378, 1396–1407 (2018).
Chapuy, B. et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat. Med. 24, 679–690 (2018).
Karube, K. et al. Integrating genomic alterations in diffuse large B-cell lymphoma identifies new relevant pathways and potential therapeutic targets. Leukemia 32, 675–684 (2018).
Arthur, S. E. et al. Genome-wide discovery of somatic regulatory variants in diffuse large B-cell lymphoma. Nat. Commun. 9, 4001 (2018).
Morin, R. D. et al. Mutational and structural analysis of diffuse large B-cell lymphoma using whole-genome sequencing. Blood 122, 1256–1265 (2013).
Schmitz, R. et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature 490, 116–120 (2012).
Lopez, C. et al. Genomic and transcriptomic changes complement each other in the pathogenesis of sporadic Burkitt lymphoma. Nat. Commun. 10, 1459 (2019).
Richter, J. et al. Recurrent mutation of the ID3 gene in Burkitt lymphoma identified by integrated genome, exome and transcriptome sequencing. Nat. Genet. 44, 1316–1320 (2012).
Panea, R. I. et al. The whole-genome landscape of Burkitt lymphoma subtypes. Blood 134, 1598–1607 (2019).
Okosun, J. et al. Integrated genomic analysis identifies recurrent mutations and evolution patterns driving the initiation and progression of follicular lymphoma. Nat. Genet. 46, 176–181 (2014).
Pasqualucci, L. et al. Genetics of follicular lymphoma transformation. Cell Rep. 6, 130–140 (2014).
Kridel, R. et al. Histological transformation and progression in follicular lymphoma: a clonal evolution study. PLoS Med. 13, e1002197 (2016).
Hahn, W. C. et al. Creation of human tumour cells with defined genetic elements. Nature 400, 464–468 (1999).
Caeser, R. et al. Genetic modification of primary human B cells to model high-grade lymphoma. Nat. Commun. 10, 4543 (2019).
Pound, J. D. & Gordon, J. Maintenance of human germinal center B cells in vitro. Blood 89, 919–928 (1997).
Arpin, C. et al. Generation of memory B cells and plasma cells in vitro. Science 268, 720–722 (1995).
Banchereau, J. et al. The CD40 antigen and its ligand. Ann. Rev. Immunol. 12, 881–922 (1994).
Kim, H. S., Zhang, X., Klyushnenkova, E. & Choi, Y. S. Stimulation of germinal center B lymphocyte proliferation by an FDC-like cell line, HK. J. Immunol. 155, 1101–1109 (1995).
Ding, B. B., Bi, E., Chen, H., Yu, J. J. & Ye, B. H. IL-21 and CD40L synergistically promote plasma cell differentiation through upregulation of Blimp-1 in human B cells. J. Immunol. 190, 1827–1836 (2013).
Linterman, M. A. et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J. Exp. Med. 207, 353–363 (2010).
Zotos, D. et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J. Exp. Med. 207, 365–378 (2010).
Christodoulopoulos, I. & Cannon, P. M. Sequences in the cytoplasmic tail of the gibbon ape leukemia virus envelope protein that prevent its incorporation into lentivirus vectors. J. Virol. 75, 4129–4138 (2001).
Mock, U., Thiele, R., Uhde, A., Fehse, B. & Horn, S. Efficient lentiviral transduction and transgene expression in primary human B cells. Hum. Gene Ther. Methods 23, 408–415 (2012).
Gong, C. et al. Sequential inverse dysregulation of the RNA helicases DDX3X and DDX3Y facilitates MYC-driven lymphomagenesis. Preprint at SSRN https://doi.org/10.2139/ssrn.3520953 (2020).
Nojima, T. et al. In-vitro derived germinal centre B cells differentially generate memory B or plasma cells in vivo. Nat. Commun. 2, 465 (2011).
Sommermann, T. et al. Functional interplay of Epstein-Barr virus oncoproteins in a mouse model of B cell lymphomagenesis. Proc. Natl Acad. Sci. USA 117, 14421–14432 (2020).
Victora, G. D. et al. Identification of human germinal center light and dark zone cells and their relationship to human B-cell lymphomas. Blood 120, 2240–2248 (2012).
Kjeldsen, M. K. et al. Multiparametric flow cytometry for identification and fluorescence activated cell sorting of five distinct B-cell subpopulations in normal tonsil tissue. Am. J. Clin. Pathol. 136, 960–969 (2011).
Kwakkenbos, M. J. et al. Generation of stable monoclonal antibody-producing B cell receptor-positive human memory B cells by genetic programming. Nat. Med. 16, 123–128 (2010).
Stevens, S. J. et al. Monitoring of epstein-barr virus DNA load in peripheral blood by quantitative competitive PCR. J. Clin. Microbiol. 37, 2852–2857 (1999).
Doench, J. G. et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat. Biotechnol. 34, 184–191 (2016).
Tzelepis, K. et al. A CRISPR dropout screen identifies genetic vulnerabilities and therapeutic targets in acute myeloid leukemia. Cell Rep. 17, 1193–1205 (2016).
Acknowledgements
D.H. was personally supported by a Clinician Scientist Fellowship from the Medical Research Council (MR/M008584/1). Research in the Hodson laboratory is supported by the Kay Kendall Leukaemia Fund, The Addenbrooke’s Charitable Trust and the Evelyn Trust. The Hodson laboratory receives core funding from Wellcome (203151/Z/16/Z) and MRC to the Wellcome-MRC Cambridge Stem Cell Institute and from CRUK to the CRUK Cambridge Centre (A25117). We thank J. Beswick, A. Mitchel and N. Jonas from the ENT Department at Addenbrooke’s Hospital, Cambridge for their assistance in the collection of primary tonsil tissue. We are grateful to K. Elston and J. Baxter from the Cambridge Blood and Stem Cell Bank for collection and storage of primary tonsils tissue and to the staff of the Central Biomedical Services for animal housing and care. We thank A. Weiss for expert technical advice. This research was supported by the Cambridge NIHR BRC Cell Phenotyping Hub and we wish to thank all members of the flow cytometry core for their advice and support in flow cytometry.
Author information
Authors and Affiliations
Contributions
R.C., J.G., M.D.R., C.G. and D.H. contributed to the development and optimization of the protocols described in this manuscript. R.C. and D.H. wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
R.C.: consultancy for Karus Therapeutics. D.H.: research funding from Gilead Sciences. The remaining authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Wolfgang Hammerschmidt and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Caeser, R. et al. Nat. Commun. 10, 4543 (2019): https://doi.org/10.1038/s41467-019-12494-x
Sommermann, T. et al. Proc. Natl Acad. Sci. USA 117, 14421–14432 (2020): https://doi.org/10.1073/pnas.1921139117
Supplementary information
Supplementary Information
Supplementary Figs. 1–3.
Source data
Source Data Fig. 4
Raw numbers.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Rights and permissions
About this article
Cite this article
Caeser, R., Gao, J., Di Re, M. et al. Genetic manipulation and immortalized culture of ex vivo primary human germinal center B cells. Nat Protoc 16, 2499–2519 (2021). https://doi.org/10.1038/s41596-021-00506-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-021-00506-4
This article is cited by
-
PARP14 is a novel target in STAT6 mutant follicular lymphoma
Leukemia (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.