Abstract
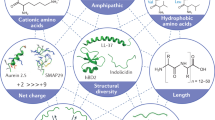
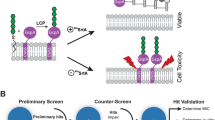
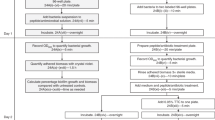
Peptides are promising drug candidates because of their diversity, biocompatibility and spectrum of activities. Here, we describe a protocol for high-throughput screening of SPOT-peptide arrays to assess the antibiofilm, antimicrobial and immunomodulatory activities of synthetic peptides. It is a Protocol Extension of our previous Nature Protocols article, which describes the synthesis of SPOT-peptide arrays and assays for screening antimicrobial activity. This latest protocol allows the simultaneous assessment of hundreds of synthetic host defense peptides to define their overall activity profiles and identify candidate sequences that are suitable for further characterization and development as anti-infectives. When coupled with the SPOT-array technology for peptide synthesis, the described procedures are rapid, inexpensive and straightforward for peptide library screening. The protocols can be implemented in most microbiology or immunology research laboratories without the need for specialists. The time to complete each step ranges between 1 and 4 h with overnight pauses, and datasets related to the antibiofilm and immunomodulatory activities of a large set of peptide sequences can be generated in a few days.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
US Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2019. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (2019).
Brown, E. D. & Wright, G. D. Antibacterial drug discovery in the resistance era. Nature 529, 336–343 (2016).
Hancock, R. E. W. The end of an era? Nat. Rev. Drug Discov. 6, 28 (2007).
MacLean, R. C. & San Millan, A. The evolution of antibiotic resistance. Science 365, 1082–1083 (2019).
O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf (2014).
The World Bank Group. Drug-Resistant Infections: A Threat to Our Economic Future. https://www.worldbank.org/en/topic/health/publication/drug-resistant-infections-a-threat-to-our-economic-future (2017).
Rudd, K. E. et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the global burden of disease study. Lancet 395, 200–211 (2020).
Harrison, J. J., Ceri, H. & Turner, R. J. Multimetal resistance and tolerance in microbial biofilms. Nat. Rev. Microbiol. 5, 928–938 (2007).
Koo, H., Allan, R. N., Howlin, R. P., Stoodley, P. & Hall-Stoodley, L. Targeting microbial biofilms: current and prospective therapeutic strategies. Nat. Rev. Microbiol. 15, 740–755 (2017).
Flemming, H. C. et al. Biofilms: an emergent form of bacterial life. Nat. Rev. Microbiol. 14, 563–575 (2016).
Mookherjee, N., Anderson, M. A., Haagsman, H. P. & Davidson, D. J. Antimicrobial host defence peptides: functions and clinical potential. Nat. Rev. Drug Discov. 19, 311–332 (2020).
Hancock, R. E. W., Haney, E. F. & Gill, E. E. The immunology of host defence peptides: beyond antimicrobial activity. Nat. Rev. Immunol. 16, 321–334 (2016).
Etayash, H., Azmi, S., Dangeti, R. & Kaur, K. Peptide bacteriocins—structure activity relationships. Curr. Top. Med. Chem. 16, 220–241 (2015).
Zhang, R. et al. Structure-function relationships of antimicrobial peptides and proteins with respect to contact molecules on pathogen surfaces. Curr. Top. Med. Chem. 16, 89–98 (2016).
de la Fuente-Nunez, C., Reffuveille, F., Haney, E. F., Straus, S. K. & Hancock, R. E. W. Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLoS Pathog. 10, e1004152 (2014).
de la Fuente-Nunez, C., Cardoso, M. H., de Souza Candido, E., Franco, O. L. & Hancock, R. E. W. Synthetic antibiofilm peptides. Biochim. Biophys. Acta 1858, 1061–1069 (2016).
Hancock, R. E. W., Nijnik, A. & Philpott, D. J. Modulating immunity as a therapy for bacterial infections. Nat. Rev. Microbiol. 10, 243–254 (2012).
Hilchie, A. L., Wuerth, K. & Hancock, R. E. W. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat. Chem. Biol. 9, 761–768 (2013).
Hancock, R. E. W. & Sahl, H. G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 24, 1551–1557 (2006).
Yeaman, M. R. & Yount, N. Y. Unifying themes in host defence effector polypeptides. Nat. Rev. Microbiol. 5, 727–740 (2007).
Etayash, H., Pletzer, D., Kumar, P., Straus, S. K. & Hancock, R. E. W. Cyclic derivative of host-defense peptide IDR-1018 improves proteolytic stability, suppresses inflammation, and enhances in vivo activity. J. Med. Chem 63, 9228–9236 (2020).
Mookherjee, N. et al. Intracellular receptor for human host defense peptide LL-37 in monocytes. J. Immunol. 183, 2688–2696 (2009).
Yu, H. B. et al. Sequestosome-1/p62 is the key intracellular target of innate defense regulator peptide. J. Biol. Chem. 284, 36007–36011 (2009).
Choi, K. Y., Chow, L. N. & Mookherjee, N. Cationic host defence peptides: multifaceted role in immune modulation and inflammation. J. Innate Immun. 4, 361–370 (2012).
Haney, E. F., Straus, S. K. & Hancock, R. E. W. Reassessing the host defense peptide landscape. Front. Chem. 7, 43 (2019).
Haney, E. F. et al. Computer-aided discovery of peptides that specifically attack bacterial biofilms. Sci. Rep. 8, 1871 (2018).
Haney, E. F., Mansour, S. C., Hilchie, A. L., de la Fuente-Nunez, C. & Hancock, R. E. W. High throughput screening methods for assessing antibiofilm and immunomodulatory activities of synthetic peptides. Peptides 71, 276–285 (2015).
Haney, E. F., Barbosa, S. C., Baquir, B. & Hancock, R. E. W. Influence of non-natural cationic amino acids on the biological activity profile of innate defense regulator peptides. J. Med. Chem. 62, 10294–10304 (2019).
Hilpert, K., Winkler, D. F. & Hancock, R. E. W. Peptide arrays on cellulose support: SPOT synthesis, a time and cost efficient method for synthesis of large numbers of peptides in a parallel and addressable fashion. Nat. Protoc. 2, 1333–1349 (2007).
Rabin, N. et al. Agents that inhibit bacterial biofilm formation. Future Med. Chem. 7, 647–671 (2015).
Haney, E. F., Trimble, M. J., Cheng, J. T., Valle, Q. & Hancock, R. E. W. Critical assessment of methods to quantify biofilm growth and evaluate antibiofilm activity of host defence peptides. Biomolecules 8, 29 (2018).
Münzker, L., Oddo, A. & Hansen, P. R. Chemical synthesis of antimicrobial peptides. Methods Mol. Biol. 1548, 35–49 (2017).
Kimmerlin, T. & Seebach, D. ‘100 years of peptide synthesis’: ligation methods for peptide and protein synthesis with applications to beta-peptide assemblies. J. Pept. Res. 65, 229–260 (2005).
Fields, G. B. Introduction to peptide synthesis. Curr. Protoc. Mol. Biol. Ch. 11, Unit 11.15 (2002).
Süssmuth, R. D. & Mainz, A. Nonribosomal peptide synthesis-principles and prospects. Angew Chem. Int. Ed. Engl. 56, 3770–3821 (2017).
Jaradat, D. M. M. Thirteen decades of peptide synthesis: key developments in solid phase peptide synthesis and amide bond formation utilized in peptide ligation. Amino Acids 50, 39–68 (2018).
Stawikowski, M. & Fields, G. B. Introduction to peptide synthesis. Curr. Protoc. Protein Sci. Ch. 18, Unit 18.11 (2012).
Coin, I., Beyermann, M. & Bienert, M. Solid-phase peptide synthesis: from standard procedures to the synthesis of difficult sequences. Nat. Protoc. 2, 3247–3256 (2007).
Behrendt, R., White, P. & Offer, J. Advances in Fmoc solid-phase peptide synthesis. J. Pept. Sci. 22, 4–27 (2016).
Mäde, V., Els-Heindl, S. & Beck-Sickinger, A. G. Automated solid-phase peptide synthesis to obtain therapeutic peptides. J. Org. Chem. 10, 1197–1212 (2014).
Merrifield, R. B. Solid-phase peptide synthesis. Adv. Enzymol. Relat. Areas Mol. Biol. 32, 221–296 (1969).
da Silva, A. Jr. et al. Avian anticoccidial activity of a novel membrane-interactive peptide selected from phage display libraries. Mol. Biochem. Parasitol. 120, 53–60 (2002).
Pini, A. et al. Antimicrobial activity of novel dendrimeric peptides obtained by phage display selection and rational modification. Antimicrob. Agents Chemother. 49, 2665–2672 (2005).
Ashby, M., Petkova, A., Gani, J., Mikut, R. & Hilpert, K. Use of peptide libraries for identification and optimization of novel antimicrobial peptides. Curr. Top Med. Chem. 17, 537–553 (2017).
Winkler, D. F., Hilpert, K., Brandt, O. & Hancock, R. E. W. Synthesis of peptide arrays using SPOT-technology and the CelluSpots-method. Methods Mol. Biol. 570, 157–174 (2009).
Winkler, D. F., Andresen, H. & Hilpert, K. SPOT synthesis as a tool to study protein-protein interactions. Methods Mol. Biol. 723, 105–127 (2011).
Kaur, K., Ahmed, S., Soudy, R. & Azmi, S. Screening peptide array library for the identification of cancer cell-binding peptides. Methods Mol. Biol. 1248, 239–247 (2015).
Soudy, R., Ahmed, S. & Kaur, K. NGR peptide ligands for targeting CD13/APN identified through peptide array screening resemble fibronectin sequences. ACS Comb. Sci. 14, 590–599 (2012).
Bluhm, M. E., Knappe, D. & Hoffmann, R. Structure-activity relationship study using peptide arrays to optimize Api137 for an increased antimicrobial activity against Pseudomonas aeruginosa. Eur. J. Med. Chem. 103, 574–582 (2015).
Knappe, D. et al. Optimization of oncocin for antibacterial activity using a SPOT synthesis approach: extending the pathogen spectrum to Staphylococcus aureus. Amino Acids 48, 269–280 (2016).
Ommen, P., Zobek, N. & Meyer, R. L. Quantification of biofilm biomass by staining: non-toxic safranin can replace the popular crystal violet. J. Microbiol. Methods 141, 87–89 (2017).
Toté, K., Vanden Berghe, D., Maes, L. & Cos, P. A new colorimetric microtitre model for the detection of Staphylococcus aureus biofilms. Lett. Appl. Microbiol. 46, 249–254 (2008).
Stiefel, P. et al. Is biofilm removal properly assessed? Comparison of different quantification methods in a 96-well plate system. Appl. Microbiol. Biotechnol. 100, 4135–4145 (2016).
Harrison, J. J. et al. Microtiter susceptibility testing of microbes growing on peg lids: a miniaturized biofilm model for high-throughput screening. Nat. Protoc. 5, 1236–1254 (2010).
Amsen, D., de Visser, K. E. & Town, T. Approaches to determine expression of inflammatory cytokines. Methods Mol. Biol. 511, 107–142 (2009).
Huang, X. et al. Effect of arginine on the growth and biofilm formation of oral bacteria. Arch. Oral Biol. 82, 256–262 (2017).
Chen, P., Abercrombie, J. J., Jeffrey, N. R. & Leung, K. P. An improved medium for growing Staphylococcus aureus biofilm. J. Microbiol. Methods 90, 115–118 (2012).
Bowdish, D. M. et al. Impact of LL-37 on anti-infective immunity. J. Leukoc. Biol. 77, 451–459 (2005).
Tomita, T. et al. Effect of ions on antibacterial activity of human beta defensin 2. Microbiol. Immunol. 44, 749–754 (2000).
Hammond, A., Dertien, J., Colmer-Hamood, J. A., Griswold, J. A. & Hamood, A. N. Serum inhibits P. aeruginosa biofilm formation on plastic surfaces and intravenous catheters. J. Surg. Res. 159, 735–746 (2010).
Wiegand, I., Hilpert, K. & Hancock, R. E. W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 3, 163–175 (2008).
Metcalf, T. U. et al. Global analyses revealed age-related alterations in innate immune responses after stimulation of pathogen recognition receptors. Aging Cell 14, 421–432 (2015).
Ter Horst, R. et al. Host and environmental factors influencing individual human cytokine responses. Cell 167, 1111–1124.e13 (2016).
Li, Y. et al. A functional genomics approach to understand variation in cytokine production in humans. Cell 167, 1099–1110.e14 (2016).
Schirmer, M., Kumar, V., Netea, M. G. & Xavier, R. J. The causes and consequences of variation in human cytokine production in health. Curr. Opin. Immunol. 54, 50–58 (2018).
Wu, B. C., Lee, A. H. & Hancock, R. E. W. Mechanisms of the innate defense regulator peptide-1002 anti-inflammatory activity in a sterile inflammation mouse model. J. Immunol. 199, 3592–3603 (2017).
Haney, E. F. et al. Identification of an IDR peptide formulation candidate that prevents peptide aggregation and retains immunomodulatory activity. Pept. Sci. (Hoboken) 111, e24077 (2019).
Martikainen, M. V. & Roponen, M. Cryopreservation affected the levels of immune responses of PBMCs and antigen-presenting cells. Toxicol. In Vitro 67, 104918 (2020).
Yang, J. et al. The effects of storage temperature on PBMC gene expression. BMC Immunol. 17, 6 (2016).
Li, X., Zhong, Z., Liang, S., Wang, X. & Zhong, F. Effect of cryopreservation on IL-4, IFNγ and IL-6 production of porcine peripheral blood lymphocytes. Cryobiology 59, 322–326 (2009).
Darveau, R. P. & Hancock, R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J. Bacteriol. 155, 831–838 (1983).
Acknowledgements
We acknowledge the financial assistance of the Canadian Institutes of Health Research, Foundation grant FDN-154287 to R.E.W.H. for funding peptide research in our laboratory. H.E. is the recipient of a UBC Killam Fellowship and a Research Trainee Award from the Michael Smith Foundation for Health Research. R.E.W.H. is a Canada Research Chair in Health and Genomics and holds a UBC Killam Professorship.
Ethics declarations
Competing interests
E.F.H. and R.E.W.H. have filed patents related to the antibiofilm and immunomodulatory functions of synthetic HDPs. These patents have been assigned to their employer, the University of British Columbia, and licensed to ABT Innovations Inc., in which R.E.W.H. has an ownership position.
Additional information
Peer review information Nature Protocols thanks Donald Davidson, Sam Walker and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Haney, E. F. et al. Sci. Rep. 8, 1871 (2018): https://doi.org/10.1038/s41598-018-19669-4
Haney, E. F., Mansour, S. C., Hilchie, A. L., de la Fuente-Nunez, C. & Hancock, R. E. W. Peptides 71, 276–285 (2015): https://doi.org/10.1016/j.peptides.2015.03.015
Haney, E. F., Barbosa, S. C., Baquir, B. & Hancock, R. E. W. J. Med. Chem. 62, 10294–10304 (2019): https://doi.org/10.1021/acs.jmedchem.9b01344
This protocol is an extension to: Nat. Protoc. 2, 1333–1349 (2007): https://doi.org/10.1038/nprot.2007.160
Supplementary information
Supplementary Information
Supplementary Table 1.
Rights and permissions
About this article
Cite this article
Etayash, H., Haney, E.F. & Hancock, R.E.W. Assessing biofilm inhibition and immunomodulatory activity of small amounts of synthetic host defense peptides synthesized using SPOT-array technology. Nat Protoc 16, 1850–1870 (2021). https://doi.org/10.1038/s41596-021-00500-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-021-00500-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.