Abstract
Background
Published health utility studies are increasingly cited in cost–utility analyses to inform reimbursement decision-making. However, there is limited guidance for investigators looking to systematically evaluate the methodological quality of health utility studies or their applicability to decision contexts.
Objective
To describe how health utility concepts are reflected in tools intended for use with the health economic literature, particularly with respect to the evaluation of methodological quality and context applicability.
Methods
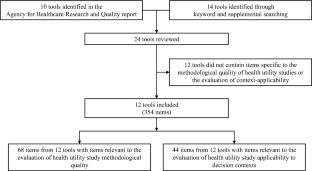
We reviewed the critical appraisal and reporting tools described in a 2012 report published by the Agency for Healthcare Research and Quality (AHRQ), supplemented with a keyword search of MEDLINE and EMBASE, to identify existing tools which include health utility constructs. From these tools, a list of relevant items was compiled and grouped into domain categories based on the methodological or applicability aspect they were directed toward.
Results
Of the 24 tools we identified, 12 contained items relevant to the evaluation of health utilities. Sixty-five items were considered relevant to the evaluation of quality, while 44 were relevant to the evaluation of applicability. Items were arranged into four domains: health state descriptions; selection and description of respondents; elicitation and measurement methods; and other considerations.
Conclusion
As key inputs to cost–utility analyses, health utilities have the potential to significantly impact estimates of cost-effectiveness. Existing tools contain only general items related to the conduct or use of health utility studies. There is a need to develop tools that systematically evaluate the methodological quality and applicability of health utility studies.
Similar content being viewed by others
References
Klarman, H.E., Francis, J.O.S., Rosenthal, G.D.: Cost Effectiveness Analysis Applied to the Treatment of Chronic Renal Disease. Med. Care 6(1), 48–54 (1968)
Bremner, K.E., Chong, C.A., Tomlinson, G., Alibhai, S.M., Krahn, M.D.: A review and meta-analysis of prostate cancer utilities. Med. Decis. Making 27(3), 288–298 (2007). https://doi.org/10.1177/0272989x07300604
Sturza, J.: A review and meta-analysis of utility values for lung cancer. Med. Decis. Making 30(6), 685–693 (2010). https://doi.org/10.1177/0272989x10369004
Paracha, N., Thuresson, P.O., Moreno, S.G., MacGilchrist, K.S.: Health state utility values in locally advanced and metastatic breast cancer by treatment line: a systematic review. Expert Rev. Pharmacoecon. Outcomes Res. 16(5), 549–559 (2016). https://doi.org/10.1080/14737167.2016.1222907
Peasgood, T., Ward, S.E., Brazier, J.: Health-state utility values in breast cancer. Expert Rev. Pharmacoecon. Outcomes Res. 10(5), 553–566 (2010). https://doi.org/10.1586/erp.10.65
Hao, Y., Wolfram, V., Cook, J.: A structured review of health utility measures and elicitation in advanced/metastatic breast cancer. Clin. Econ. Outcomes Res. CEOR 8, 293–303 (2016). https://doi.org/10.2147/ceor.s100448
Schiller-Fruhwirth, I.C., Jahn, B., Arvandi, M., Siebert, U.: Cost-effectiveness models in breast cancer screening in the general population: a systematic review. Appl. Health Econ. Health Policy 15(3), 333–351 (2017). https://doi.org/10.1007/s40258-017-0312-3
Carter, G.C., King, D.T., Hess, L.M., Mitchell, S.A., Taipale, K.L., Kiiskinen, U., Rajan, N., Novick, D., Liepa, A.M.: Health state utility values associated with advanced gastric, oesophageal, or gastro-oesophageal junction adenocarcinoma: a systematic review. J. Med. Econ. 18(11), 954–966 (2015). https://doi.org/10.3111/13696998.2015.1066380
Djalalov, S., Rabeneck, L., Tomlinson, G., Bremner, K.E., Hilsden, R., Hoch, J.S.: A review and meta-analysis of colorectal cancer utilities. Med. Decis. Making 34(6), 809–818 (2014). https://doi.org/10.1177/0272989x14536779
Jeong, K., Cairns, J.: Systematic review of health state utility values for economic evaluation of colorectal cancer. Heal. Econ. Rev. 6(1), 36 (2016). https://doi.org/10.1186/s13561-016-0115-5
Richardson, J., Khan, M.A., Iezzi, A., Maxwell, A.: Comparing and explaining differences in the magnitude, content, and sensitivity of utilities predicted by the EQ-5D, SF-6D, HUI 3, 15D, QWB, and AQoL-8D multiattribute utility instruments. Med. Decis. Making 35(3), 276–291 (2015). https://doi.org/10.1177/0272989x14543107
Dolan, P.: Modeling valuations for EuroQol health states. Med. Care 35(11), 1095–1108 (1997). https://doi.org/10.1097/00005650-199711000-00002
Mulhern, B.J., Bansback, N., Norman, R., Brazier, J.: Valuing the SF-6Dv2 classification system in the United Kingdom using a discrete-choice experiment with duration. Med. Care 58(6), 566–573 (2020). https://doi.org/10.1097/mlr.0000000000001324
Galante, J., Augustovski, F., Colantonio, L., Bardach, A., Caporale, J., Marti, S.G., Kind, P.: Estimation and comparison of EQ-5D health states' utility weights for pneumoccocal and human papillomavirus diseases in Argentina, Chile, and the United Kingdom. Value Health 14(5, Supplement), S60–S64 (2011). https://doi.org/10.1016/j.jval.2011.05.007
Takemoto, M.L., Lopes da Silva, N., Ribeiro-Pereira, A.C., Schilithz, A.O., Suzuki, C.: Differences in utility scores obtained through Brazilian and UK value sets: a cross-sectional study. Health Qual. Life Outcomes 13, 119 (2015). https://doi.org/10.1186/s12955-015-0318-1
Pollard, C., Hartz, S., Leage, S.L., Paget, M.A., Cook, J., Enstone, A.: Elicitation of health state utilities associated with varying severities of flares in systemic lupus erythematosus. Health Qual. Life Outcomes 13, 66 (2015). https://doi.org/10.1186/s12955-015-0262-0
Brazier, J., Ara, R., Azzabi, I., Busschbach, J., Chevrou-Severac, H., Crawford, B., Cruz, L., Karnon, J., Lloyd, A., Paisley, S., Pickard, A.S.: Identification, review, and use of health state utilities in cost-effectiveness models: an ISPOR Good practices for outcomes research task force report. Value Health 22(3), 267–275 (2019). https://doi.org/10.1016/j.jval.2019.01.004
Xie, F., Zoratti, M., Chan, K., Husereau, D., Krahn, M., Levine, O., Clifford, T., Schunemann, H., Guyatt, G.: Toward a centralized, systematic approach to the identification, appraisal, and use of health state utility values for reimbursement decision making: introducing the health utility book (HUB). Med. Decis. Making 39(4), 370–378 (2019). https://doi.org/10.1177/0272989x19837969
Walker, D.G., Wilson, R.F., Sharma, R., Bridges, J., Niessen, L., Bass, E.B., Frick, K.: Best practices for conducting economic evaluations in health care: a systematic review of quality assessment tools. AHRQ Publication No. 12(13)-EHC132-EF. Agency for Healthcare Research and Quality (2012)
Husereau, D., Drummond, M., Petrou, S., Carswell, C., Moher, D., Greenberg, D., Augustovski, F., Briggs, A.H., Mauskopf, J., Loder, E.: Consolidated health economic evaluation reporting standards (CHEERS)–explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value Health 16(2), 231–250 (2013). https://doi.org/10.1016/j.jval.2013.02.002
Evers, S., Goossens, M., de Vet, H., van Tulder, M., Ament, A.: Criteria list for assessment of methodological quality of economic evaluations: consensus on health economic criteria. Int. J. Technol. Assess. Health Care 21(2), 240–245 (2005)
Nerich, V., Saing, S., Gamper, E.M., Holzner, B., Pivot, X., Viney, R., Kemmler, G.: Critical appraisal of health-state utility values used in breast cancer-related cost-utility analyses. Breast Cancer Res. Treat. (2017). https://doi.org/10.1007/s10549-017-4283-8
Chiou, C.F., Hay, J.W., Wallace, J.F., Bloom, B.S., Neumann, P.J., Sullivan, S.D., Yu, H.T., Keeler, E.B., Henning, J.M., Ofman, J.J.: Development and validation of a grading system for the quality of cost-effectiveness studies. Med. Care 41(1), 32–44 (2003). https://doi.org/10.1097/01.mlr.0000039824.73620.e5
Simoens, S.: Assessment of methodological quality of economic evaluations in belgian drug reimbursement applications. PLoS One 8(12), e85411 (2013). https://doi.org/10.1371/journal.pone.0085411
Stalmeier, P.F., Goldstein, M.K., Holmes, A.M., Lenert, L., Miyamoto, J., Stiggelbout, A.M., Torrance, G.W., Tsevat, J.: What should be reported in a methods section on utility assessment? Med. Decis. Making 21(3), 200–207 (2001)
Petrou, S., Rivero-Arias, O., Dakin, H., Longworth, L., Oppe, M., Froud, R., Gray, A.: Preferred reporting items for studies mapping onto preference-based outcome measures: the MAPS statement. Pharmacoeconomics 33(10), 985–991 (2015). https://doi.org/10.1007/s40273-015-0319-2
Xie, F., Pickard, A.S., Krabbe, P.F., Revicki, D., Viney, R., Devlin, N., Feeny, D.: A checklist for reporting valuation studies of multi-attribute utility-based instruments (CREATE). Pharmacoeconomics 33(8), 867–877 (2015). https://doi.org/10.1007/s40273-015-0292-9
Brazier, J., Deverill, M., Green, C.: A review of the use of health status measures in economic evaluation. J. Health Serv. Res. Policy 4(3), 174–184 (1999). https://doi.org/10.1177/135581969900400310
Drummond, M.F., Jefferson, T.O.: Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 313(7052), 275–283 (1996)
Ungar, W.J., Santos, M.T.: The pediatric quality appraisal questionnaire: an instrument for evaluation of the pediatric health economics literature. Value Health 6(5), 584–594 (2003). https://doi.org/10.1046/j.1524-4733.2003.65253.x
Clemens, K., Townsend, R., Luscombe, F., Mauskopf, J., Osterhaus, J., Bobula, J.: Methodological and conduct principles for pharmacoeconomic research. Pharmaceutical Research and Manufacturers of America. PharmacoEconomics 8(2), 169–174 (1995)
Adams, M.E., McCall, N.T., Gray, D.T., Orza, M.J., Chalmers, T.C.: Economic analysis in randomized control trials. Med. Care 30(3), 231–243 (1992)
Gerard, K.: Cost-utility in practice: a policy maker’s guide to the state of the art. Health Policy 21(3), 249–279 (1992)
Sacristan, J.A., Soto, J., Galende, I.: Evaluation of pharmacoeconomic studies: utilization of a checklist. Ann. Pharmacother. 27(9), 1126–1133 (1993). https://doi.org/10.1177/106002809302700919
Drummond, M., Manca, A., Sculpher, M.: Increasing the generalizability of economic evaluations: recommendations for the design, analysis, and reporting of studies. Int. J. Technol. Assess. Health Care 21(2), 165–171 (2005)
Ramsey, S., Willke, R., Briggs, A., Brown, R., Buxton, M., Chawla, A., Cook, J., Glick, H., Liljas, B., Petitti, D., Reed, S.: Good research practices for cost-effectiveness analysis alongside clinical trials: the ISPOR RCT-CEA Task Force report. Value Health 8(5), 521–533 (2005). https://doi.org/10.1111/j.1524-4733.2005.00045.x
Goetghebeur, M.M., Wagner, M., Khoury, H., Levitt, R.J., Erickson, L.J., Rindress, D.: Evidence and value: Impact on decisionMaking–the EVIDEM framework and potential applications. BMC Health Serv. Res. 8, 270 (2008). https://doi.org/10.1186/1472-6963-8-270
Davis, J.C., Robertson, M.C., Comans, T., Scuffham, P.A.: Guidelines for conducting and reporting economic evaluation of fall prevention strategies. Osteoporos Int. 22(9), 2449–2459 (2011). https://doi.org/10.1007/s00198-010-1482-0
Vintzileos, A.M., Beazoglou, T.: Design, execution, interpretation, and reporting of economic evaluation studies in obstetrics. Am. J. Obstet. Gynecol. 191(4), 1070–1076 (2004). https://doi.org/10.1016/j.ajog.2004.05.021
Grutters, J.P., Seferina, S.C., Tjan-Heijnen, V.C., van Kampen, R.J., Goettsch, W.G., Joore, M.A.: Bridging trial and decision: a checklist to frame health technology assessments for resource allocation decisions. Value Health 14(5), 777–784 (2011). https://doi.org/10.1016/j.jval.2011.01.005
Russell, L.B., Gold, M.R., Siegel, J.E., Daniels, N., Weinstein, M.C.: The role of cost-effectiveness analysis in health and medicine. Panel on cost-effectiveness in health and medicine. Jama 276(14), 1172–1177 (1996)
Siegel, J.E., Weinstein, M.C., Russell, L.B., Gold, M.R.: Recommendations for reporting cost-effectiveness analyses. Panel on cost-effectiveness in health and medicine. Jama 276(16), 1339–1341 (1996)
Weinstein, M.C., Siegel, J.E., Gold, M.R., Kamlet, M.S., Russell, L.B.: Recommendations of the panel on cost-effectiveness in health and medicine. JAMA 276(15), 1253–1258 (1996)
Sanders, G.D., Neumann, P.J., Basu, A., Brock, D.W., Feeny, D., Krahn, M., Kuntz, K.M., Meltzer, D.O., Owens, D.K., Prosser, L.A., Salomon, J.A., Sculpher, M.J., Trikalinos, T.A., Russell, L.B., Siegel, J.E., Ganiats, T.G.: Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA 316(10), 1093–1103 (2016). https://doi.org/10.1001/jama.2016.12195
Higgins, J.P.T., Altman, D.G., Gøtzsche, P.C., Jüni, P., Moher, D., Oxman, A.D., Savović, J., Schulz, K.F., Weeks, L., Sterne, J.A.C.: The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. BMJ 343 (2011). https://doi.org/10.1136/bmj.d5928
Mokkink, L.B., Terwee, C.B., Patrick, D.L., Alonso, J., Stratford, P.W., Knol, D.L., Bouter, L.M., de Vet, H.C.: The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Quality Life Res. 19(4), 539–549 (2010). https://doi.org/10.1007/s11136-010-9606-8
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
None to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zoratti, M.J., Pickard, A.S., Stalmeier, P.F.M. et al. Evaluating the conduct and application of health utility studies: a review of critical appraisal tools and reporting checklists. Eur J Health Econ 22, 723–733 (2021). https://doi.org/10.1007/s10198-021-01286-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-021-01286-0




