Abstract
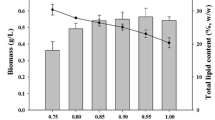
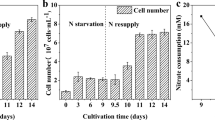
To study the effects of exogenous metabolic intermediates and antioxidant on Nannochloropsis oculata microalgae strain, the growth performance, biomass productivity, photosynthetic efficiency, and total lipid content were investigated by lab-scale cultivation experiments. Specifically, N. oculata was cultured in f/2 medium supplemented with different concentrations of citric acid or malic acid (0.050, 0.150, 0.750 g L−1) and KI (0.025, 0.050, 0.100, and 0.500 g L−1) to examine the physiological and biochemical effects on the widely distributed marine algae N. oculata. Results showed that the biomass and photosynthetic efficiency showed a downward trend with increasing citric acid and malic acid concentrations, and the low concentrations of metabolic intermediate treatments could enhance the lipid accumulation. The highest lipid content (0.295 or 0.245 g total lipid per gram of dry biomass) was found in cells treated with 0.15 g L-1 of citric acid and malic acid, which was about 28.45% and 6.99% higher than that growing in the culture lacking metabolic intermediates. Compared with malic acid, citric acid had better effect. Furthermore, potassium iodide (KI), an antioxidant, could also boost the lipid content of N. oculata with slight declining biomass productivity, and the highest lipid content (0.300 g total lipid per gram of dry biomass) was found in cells treated with 0.100 g L-1 of KI, which was about 28.08% higher than that growing in the culture lacking KI, indicating that KI may be a potential factor to increase the lipid content of algae cells. These findings not only provide insight into the response mechanism of N. oculata to exogenous metabolic intermediates and antioxidant but also help further broaden the key regulatory factors involved in the lipid accumulation within algal cells.







Similar content being viewed by others
Abbreviations
- OD680 :
-
Optical density 680 nm
- Fv/Fm:
-
Maximum fluorescence
- PSII:
-
Photosystem II
- W k :
-
The damage degree of the donor side of PSII reaction center under exogenous stress
- ABS/RC:
-
Absorption flux per reaction center
- DIo/RC:
-
Dissipated energy flux per reaction center
- ETo/RC:
-
Electron transport per reaction center
- TRo/RC:
-
Trapped energy flux per reaction center
- ETo/ABS:
-
Quantum yield for electron transport
- ETo/TRo:
-
The probability that a trapped exciton moves an electron into the electron transport chain beyond QA
- Mo:
-
The rate at which QA is reduced
- Sm:
-
Normalized total complementary area above the O-J-I-P curve
- V J :
-
Relative variable fluorescence intensity at the J-step
References
Chiari L, Zecca A (2011) Constraints of fossil fuels depletion on global warming projections. Energ Policy 39(9):5026–5034
Höök M, Tang X (2013) Depletion of fossil fuels and anthropogenic climate change-a review. Energ Policy 52:797–809
Cutz L, Haro P, Santana D, Johnsson F (2016) Assessment of biomass energy sources and technologies: the case of Central America. Renew Sust Energ Rev 58:1411–1431
Demirbas A (2009) Progress and recent trends in biodiesel fuels. Energ Convers Manage 50(1):14–34
Papanikolaou S, Aggelis G (2011) Lipids of oleaginous yeasts. Part II: technology and potential applications. Eur J Lipid Sci Technol 113(8):1052–1073
Poontawee R, Yongmanitchai W, Limtong S (2017) Efficient oleaginous yeasts for lipid production from lignocellulosic sugars and effects of lignocellulose degradation compounds on growth and lipid production. Process Biochem 53:44–60
Wijffels RH, Barbosa MJ (2010) An outlook on microalgal biofuels. Science 329(5993):796–799
Makareviciene V, Skorupskaite V, Andruleviciute V (2013) Biodiesel fuel from microalgae-promising alternative fuel for the future: a review. Rev Environ Sci Biotechnol 12(2):119–130
Raheem A, Azlina WAKGW, Yap YHT, Danquah MK, Harun R (2015) Thermochemical conversion of microalgal biomass for biofuel production. Renew Sust Energ Rev 49:990–999
Chen H, Qiu T, Rong JF, He CL, Wang Q (2015) Microalgal biofuel revisited: an informatics-based analysis of developments to date and future prospects. Appl Energy 155:585–598
Deng XD, Gu B, Li YJ, Hu XW, Guo JC, Fei XW (2012) The roles of acyl-CoA: diacylglycerol acyltransferase 2 genes in the biosynthesis of triacylglycerols by the green algae Chlamydomonas reinhardtii. Mol Plant 5(4):945–947
De Bhowmick G, Koduru L, Sen R (2015) Metabolic pathway engineering towards enhancing microalgal lipid biosynthesis for biofuel application—a review. Renew Sust Energ Rev 50:1239–1253
Li YT, Han DX, Hu GR, Sommerfeld M, Hu Q (2010) Inhibition of starch synthesis results in overproduction of lipids in Chlamydomonas reinhardtii. Biotechnol Bioeng 107(2):258–268
Trentacoste EM, Shrestha RP, Smith SR, Gle C, Hartmann AC, Hildebrand M, Gerwick WH (2013) Metabolic engineering of lipid catabolism increases microalgal lipid accumulation without compromising growth. P Natl Sci USA 110(49):19748–19753
Pancha I, Chokshi K, George B, Ghosh T, Paliwal C, Maurya R, Mishra S (2014) Nitrogen stress triggered biochemical and morphological changes in the microalgae Scenedesmus sp. CCNM 1077. Bioresour Technol 156:146–154
Pancha I, Chokshi K, Mishra S (2015) Enhanced biofuel production potential with nutritional stress amelioration through optimization of carbon source and light intensity in Scenedesmus sp. CCNM 1077. Bioresour Technol 179:565–572
George B, Pancha I, Desai C, Chokshi K, Paliwal C, Ghost T, Mishra S (2014) Effects of different media composition, light intensity and photoperiod on morphology and physiology of freshwater microalgae Ankistrodesmus falcatus–a potential strain for bio-fuel production. Bioresour Technol 171:367–374
Ji X, Cheng J, Gong DH, Zhao XJ, Qi Y, Su YN, Ma WC (2018) The effect of NaCl stress on photosynthetic efficiency and lipid production in freshwater microalga—Scenedesmus obliquus XJ002. Sci Total Environ 633:593–599
Monteiro CM, Fonseca SC, Castro PML, Malcata FX (2011) Toxicity of cadmium and zinc on two microalgae, Scenedesmus obliquus and Desmodesmus pleiomorphus, from Northern Portugal. J Appl Phycol 23(1):97–103
Cheng J, Du XY, Long HY, Zhang H, Ji X (2020) The effects of exogenous cerium on photosystem II as probed by in vivo chlorophyll fluorescence and lipid production of Scenedesmus obliquus XJ002. Biotechnol Appl Biochem. https://doi.org/10.1002/bab.2043
Kim DG, Lee C, Park SM, Choi YE (2014) Manipulation of light wavelength at appropriate growth stage to enhance biomass productivity and fatty acid methyl ester yield using Chlorella vulgaris. Bioresour Technol 159:240–248
Lindermayr C, Durner J (2015) Interplay of reactive oxygen species and nitric oxide: nitric oxide coordinates reactive oxygen species homeostasis. Plant Physiol 167(4):1209–1210
Seto A, Kumasaka K, Hosaka M, Kosaka E, Kashiwakura M (1992) Production of eicosapentaenoic acid by a marine microalgae and its commercial utilization for aquaculture. J Am Oil Chem Soc 1:441–456
Abdelghany MF, El-Sawy HB, Abd El-hameed SAA, Khames MK, Abdel-Latife HMR, Naielf MAE (2020) Effects of dietary Nannochloropsis oculata on growth performance, serum biochemical parameters, immune responses, and resistance against Aeromonas veronii challenge in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 107:277–288
Chernikova TN, Bargiela R, Toshchakov SV, Shivaraman V, Lunev EA, Yakimov MM, Thomas DN, Golyshin PN (2020) Hydrocarbon-degrading bacteria Alcanivorax and Marinobacter associated with microalgae Pavlova lutheri and Nannochloropsis oculata. Front microbial 11:2650–2663
Matsui H, Shiozaki K, Okumura Y, Ishikawaa M, Waqalevu V, Hayasaka O, Honda A, Kotani T (2020) Effects of phosphorous deficiency of a microalga Nannochloropsis oculata on its fatty acid profiles and intracellular structure and the effectiveness in rotifer nutrition. Algal Res 49:101905–101915
Sabzi S, Mehrgan MS, Islami HR, Shekarabi SPH (2021) Changes in biochemical composition and fatty acid accumulation of Nannochloropsis oculata in response to different iron concentrations. Biofuels 12:1–7
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37(8):911–917
Ji X, Cheng J, Gong DH, Zhao XJ, Qi Y, Su YN, Ma WC (2018) The effect of NaCl stress on photosynthetic efficiency and lipid production in freshwater microalga—Scenedesmus obliquus XJ002[J]. Sci Total Environ 633:593–599
Morant-Manceau A, Pradier E, Tremblin G (2004) Osmotic adjustment, gas exchanges and chlorophyll fluorescence of a hexaploid triticale and its parental species under salt stress. J Plant Physiol 161(1):25–33
Li P, Cheng L, Gao H, Jiang C, Peng T (2009) Heterogeneous behavior of PSII in soybean (Glycine max) leaves with identical PSII photochemistry efficiency under different high temperature treatments. J Plant Physiol 166(15):1607–1615
Guo H, Yao JT, Sun ZM, Duan DL (2015) Effects of salinity and nutrients on the growth and chlorophyll fluorescence of Caulerpa lentillifera. Chin J Oceanol Limnol 33(2):410–418
Zimmermann MB (2008) Iodine deficiency in pregnancy and the effects of maternal iodine supplementation on the offspring: a review. Am J Clin Nutr 89(2):668S–672S
Acknowledgements
Jie Cheng wants to thank, in particular, for the patience, care, and support received from Xiongyan Du over the years. Our deepest gratitude goes to the editors and anonymous reviewers for their careful work and thoughtful suggestions that have helped improve this paper substantially. This paper is a gift to the centenary celebration of Xiamen University to be held on April 6, 2021.
Author information
Authors and Affiliations
Contributions
Jie Cheng conceived and designed the project. Jie Cheng and Linggang Zheng performed the experiment and analyzed the data. Wenxin Fan assisted with the data collection and performed constructive discussions. Jie Cheng wrote the manuscript. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlight
• Changes in biomass, photosynthetic efficiency, and total lipid content of N. oculata in response to metabolic intermediates and antioxidant were investigated.
• The biomass and photosynthetic efficiency showed a downward trend with increasing citric acid and malic acid concentrations.
• The low concentrations of metabolic intermediate treatments could enhance the lipid accumulation.
• Potassium iodide could also boost the lipid content of N. oculata with slight declining biomass.
Rights and permissions
About this article
Cite this article
Cheng, J., Fan, W. & Zheng, L. Changes in biomass, photosynthetic efficiency, and total lipid content of Nannochloropsis oculata in response to metabolic intermediates and antioxidant. Biomass Conv. Bioref. 13, 5035–5042 (2023). https://doi.org/10.1007/s13399-021-01485-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01485-y