Abstract
A tapping process model of the steel from the basic oxygen furnace (BOF) addressing the reactions in the ladle is proposed. In the model, the effective equilibrium reaction zone (EERZ) method is applied to describe the steel/slag interfacial reaction. The equilibrium reactions in the bulk steel (steel/inclusion/lining wear) and slag (liquid slag/slag additions/lining wear) are considered. The thermodynamic library—ChemApp is used to perform thermodynamic calculation. The process model includes most of the actions during the tapping process, such as the additions of ferroalloys and slag formers, carryover slag entrapment and air pick-up. After the calibration by the industrial measurements of two plants, the model is applied to study the influence of the amount of carryover slag.
Similar content being viewed by others
Introduction
Steel tapping from the basic oxygen furnace (BOF) into the ladle is the critical first step in ladle treatment. Deoxidation and alloying start during the tapping process, resulting in the generation of the primary inclusions and affecting the subsequent inclusion control. The refining slag is formed from the addition of slag formers, BOF carryover slag and separated inclusions during steel tapping. Steelmakers intend to minimize the amount of BOF carryover slag due to its negative effects. The chemical composition and qualities of the refining slag further influence the steel composition and cleanliness due to the interfacial reaction. The tapping process is a kind of ‘black box’, as information from samples before the start of ladle refining is rarely available. To effectively control the steel refining process and achieve high quality steel, it is important and necessary to track the reactions and composition changes in the steel, slag and inclusions during the tapping process.
Modeling simulation is an effective method to study the reactions in the steel tapping and refining process. Galindo et al.[1] proposed a thermodynamic model on steel deoxidation processes during tapping and refining. In the model, the thermodynamic equilibrium of inclusion and metal, inclusion and entrapped slag by steel and mass balance were considered, while the interfacial reaction of steel and slag was not accounted for. The model predictions suggested that the addition sequence of alloys played an important role regarding the amount and types of inclusions. A similar model of the tapping process and its applications in deoxidation practice was presented by Cicutti and Capurro.[2] Besides the thermodynamic reactions, the fluid flow of steel and alloy additions and alloy dissolution in the tapping ladle were studied separately.[3,4] However, comprehensive modeling work on the reactions of the tapping process is missing. A variety of models of ladle refining processes have been reported, as summarized in the former publication, which offered valuable references for the simulation of the tapping process.[5]
The present project aims at modeling the entire steel refining process from liquid steel tapping and refining to the solidification to track the changes in the steel, slag and inclusions. It is believed that the comprehensive model is gaining more importance because each of the metallurgical factors in the process can affect the steel quality and production. The basic concept of the project is to link metallurgical models to a thermodynamic database. In the former study, microsegregation and inclusion formation during the cooling and solidification process of steel were simulated;[6,7,8,9] the ladle refining and Ruhrstahl–Heraeus (RH) models were constructed and validated through comparing the reported industrial practice.[5,10]
For the present study, a comprehensive model of the steel tapping process addressing the reactions in the ladle was proposed. In the model, thermodynamic equilibrium calculations were performed using thermodynamic library—ChemApp.[11] The effective equilibrium reaction zone (EERZ) method was applied to describe the steel/slag interfacial reaction.[12,13] The equilibrium reactions in the bulk steel (steel/inclusion/lining wear) and slag (liquid slag/slag additions/lining wear) are considered. Most of the actions, such as the additions of ferroalloys and slag formers, carryover slag entrapment and air pick-up, were included. The model was calibrated by comparing the predicted compositions of the steel and slag with the measurements from a total of 10 heats from two plants. The composition developments of the steel, slag and inclusions of an example case were presented. The influence of the amount of carryover slag was studied using the calibrated model.
Modeling
Following the concept of the project, the proposed tapping process modeling work applied ChemApp to link the metallurgical model to the thermodynamic database of FactSage. FactSage and ChemApp are products of GTT Technologies, Herzogenrath, Germany. The program language of the model is FORTRAN.
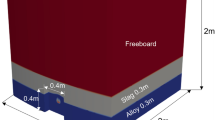
The schematic of the present model is displayed in Figure 1. In the tapping process of steel, liquid steel with a mass of \( \Delta m_{\text{BOF-st}} \) (Eq. [1]) is tapped from the BOF to the ladle at each time step (\( \Delta t \)). The tapping flow rate (\( V_{st} \)) is calculated in Eq. [2]. The steel tapping results in turbulent mixing of steel, carryover slag, added slag formers and alloying additions. The EERZ method is applied to describe the steel/slag interfacial reaction. The validation of this method for treating the interfacial reaction has been illustrated by several applications from different authors.[14,15,16,17] In the EERZ method, an effective equilibrium reaction zone containing both steel and slag phases (the region between the dashed lines in Figure 1) is defined. The mass of steel (\( \Delta m_{\text{st}} \)) and slag (\( \Delta m_{\text{sl}} \)) present in the interfacial reaction per time step is calculated based on the mass transfer coefficient (\( k_{\text{st}} \;{\text{and}}\;k_{\text{sl}} \)), as given in Eqs. [3] and [4]. The mass transfer coefficient of steel is an adjustable parameter and calibrated based on plant measurements. The mass transfer coefficient of slag is assumed to be one tenth of that of steel.[18] The steel and slag phases return to the bulk phase after the interfacial reaction and approach thermodynamic equilibrium, respectively. As mentioned above, thermodynamic equilibrium calculation is carried out using ChemApp. The inclusions formed are assumed to be in equilibrium state with bulk steel and floated at a time-independent, constant rate (\( r_{\text{fl}} \)) to the interface. The inclusion floating rate is an adjustable parameter and parametrized by the total oxygen and Al content measured. The mass of the inclusions present in the interfacial reaction at each time step is calculated by Eq. [5]. The gas phase generated is assumed to be completely removed from the system.
As shown in Figure 1, BOF carryover slag is added to the bulk slag during the steel tapping. The total amount of the carryover slag is estimated based on the overall mass balance. The total slag carryover is divided into pre-slag, vortex slag and post slag; the relevant proportions of total carryover slag depend on the tapping practice of a steel plant.[19,20] It is assumed that the pre-slag (\( m_{\text{pre-sl}} \)) is added to the ladle before tapping; the vortex slag is added in a mass of \( \Delta m_{\text{BOF-sl}} \) (Eqs. [6] and [7]) at each calculation step; the post slag (\( m_{\text{post-sl}} \)) is added after the end of tapping. The chemical composition of the BOF slag and the proportion of the partial amount of carryover slag are defined according to the data and experience of the particular plant. Besides the carryover slag, slag formers such as lime and magnesium oxide are presumed to be added to and dissolved in the bulk slag at a constant dissolution rate. The dissolution rate for all slag formers (\( V_{\text{sl}} \)) is assumed as 1 kg/s. Note that the dissolution rates of the slag formers and alloys are only the first assumptions and will have to be parameterized in the future. The mass of the slag former that enters into the system at each time step is calculated by means of Eq. [8].
For alloy additions, it is assumed that graphite and Al as buoyant alloys float to the surface of the steel after addition and thus, they are added to the steel/slag interfacial zone.[21] The alloys with comparably higher density such as FeSi, FeMn and FeCr are added to the bulk steel. During the addition, the alloys can be oxidized by atmosphere. The recovery rate defines the ratio of the alloying elements dissolving in the steel (\( r_{\text{re-X}} \)) and oxidizing during addition (\( 1 - r_{\text{re-X}} \)). The particular oxidation product (except the gas phase, which is removed from the system) is added to the bulk slag. The total mass of the alloy added to the steel and the corresponding oxidation products are calculated using Eqs. [9] and [10], respectively. At each time step, the mass of one alloy and the corresponding oxidation products is \( \Delta m_{\text{X}} \) and \( \Delta m_{\text{XO}} \) as given in Eqs. [11] and [12]. The alloy is assumed to be added and dissolved into the steel at a constant dissolution rate. The time period of alloying addition and the dissolution rate are defined individually for each alloy (\( V_{\text{X}} \)): 6 kg/s for Al and 1.5 kg/s for the others which are only the first estimates due to lack of published data. The dissolution rate of the oxidation products (\( V_{\text{XO}} \)) is calculated dissolution rate of the alloy and its compositions. Note that the impurity elements in the alloy—except Fe—are assumed to have the same recovery rate as the alloy; all Fe in the alloy is added to the steel. Taking the addition of the FeMn alloy with a mass of \( m_{FeMn} \) and a composition of 78 pct Mn, 14 pct Fe and 8 pct C as an example: the mass of Mn, Fe and C added to the steel is calculated using Eqs. [13] through [15]; the mass of MnO as the oxidation product is given in Eq. [16]. In the present model, at each time step, the mass of lining wear added into the steel (\( \Delta m_{\text{lin-st}} \)) and slag (\( \Delta m_{\text{lin-sl}} \)) as well as air pick-up \( (\Delta m_{\text{O}} \;{\text{and}}\;\Delta m_{\text{N}} ) \) by the steel stream is simply treated by rate parameters, as presented in Eqs. [17] through [20]. These corresponding rate parameters can be defined and calibrated based on the mass balance of the industrial process and measurements. Note that lining wear is added as the main oxide components: Al2O3 is added to the steel and MgO is added to the slag; other components are also considered. The gas phase generated is assumed to be out of the system. The temperature in the system is considered as prescribed according to the process data; the heat is not balanced.
where \( \Delta m_{\text{BOF-st}} \) is the mass of steel tapped from the BOF into the ladle at each time step \( \Delta t \); \( V_{\text{st}} \) is the tapping rate of steel; \( m_{\text{BOF-st}} \) is the tapping mass of the BOF steel; \( t_{\text{tap}} \) is the tapping time; subscripts of \( {\text{st}} \), \( {\text{sl}} \) and \( {\text{incl}} \) mean steel, slag and inclusion in Eqs. [3] through [5], respectively; \( \Delta m \) is the mass present in the interfacial reaction at each time step \( \Delta t \); k denotes the mass transfer coefficient; A is the interface area of the steel and slag; \( \rho \) is the density; \( r_{fl} \) is the inclusion floating rate;\( \Delta m_{\text{BOF-sl}} \) is the mass of the vortex slag added at each time step; \( V_{\text{BOF-sl}} \) is the cover rate of the vortex BOF slag during tapping; \( m_{\text{vor-sl}} \) is the mass of the vortex BOF slag; \( \Delta m_{\text{slX}} \) is the dissolved mass of slag former X at each calculation step; \( V_{\text{sl}} \) is the dissolution rate of the slag formers; in Eqs. [9] through [12], X, O and XO stand for alloy X, oxygen O and the oxide XO formed during alloying; \( m \) and \( m_{\text{st}} \) are the total mass and the mass dissolved into the steel; \( r_{\text{re-X}} \) is the recovery rate of alloy X; \( M \) is the molar mass; \( \Delta m \) and \( {\text{V}} \) are the dissolved mass and dissolution rate at each time step; \( m_{\text{Mn-FeMn}} \), \( m_{\text{Fe-FeMn}} \) and \( m_{\text{C-FeMn}} \) are the mass of Mn, Fe and C added to the steel when adding the FeMn alloy; \( m_{\text{FeMn}} \) is the mass of the FeMn alloy added; \( r_{\text{re-FeMn}} \) is the recovery rate of the FeMn alloy; \( m_{\text{MnO-FeMn}} \) is the mass of MnO, which is the oxidation product of the FeMn addition; \( M_{\text{Mn}} \) and \( M_{\text{MnO}} \) are the molar mass of Mn and MnO, respectively; O, N, \( {\text{lin-st}} \) and \( {\text{lin-sl}} \) mean oxygen, nitrogen, lining dissolution into the steel and slag in Eqs. [17] through [20]; \( \Delta m \) and \( r \) are the added mass in each time step and rate parameter.
Figure 2 shows the calculation flow chart of the tapping process model. The mass and compositions of the BOF steel and carryover slag, temperature, the time schedule of the tapping, alloy and slag former additions and alloy compositions are input into the program as the boundary conditions. With every time step, the mass of the removed inclusions (\( \Delta m_{\text{incl}} \)), buoyant alloys, steel (\( \Delta m_{\text{st}} \)) and slag (\( \Delta m_{\text{sl}} \)) transferred into the interfacial reaction zone are defined and the multiphase equilibrium calculation is performed. Afterwards, the liquid steel and slag products are returned to the bulk steel and slag, respectively. A small amount of liquid slag product (the entrapped slag) is entrapped by the liquid steel product and transferred to the bulk steel as inclusion source. The newly tapped steel and alloy additions are included in the bulk steel equilibrium calculations. The carryover slag, slag former addition and oxidized alloys are considered in the bulk slag equilibrium calculation. The equilibrium bulk steel and slag as well as the inclusion phase are ready for the calculation of the next step. Note that the formed gas phase in the steel/slag reaction and bulk steel are removed from the system, while the mass and composition of the gas phase are recorded. In the present calculations, FSstel, FToxid, and FactPS database from FactSage 7.3 were selected and applied. The LIQUID phase from FSstel is used to describe the liquid steel. The Slag–liquid phase from FToxid is applied to describe the liquid slag and liquid inclusion in the steel. Meanwhile, the following phases: FeO(s) from FactPS, CaS from FSstel and MgO, Al2O3, SiO2, P2O5, CaO, MnO, Cr2O3, Fe2O3, Mn2O3, MgAl2O4, CaAl2O4, CaAl4O7, CaAl2SiO6 and CaAl12O19 from FToxid are used to describe the precipitations in the liquid steel and slag. The time step is set as 5 seconds during tapping.
Model Calibration
To calibrate the adjustable parameters and validate the proposed model, 10 heats of industrial experiments were carried out in two plants (5 heats at Plant A and 5 heats at Plant B). The tapping schedules of the experimental heats at each plant were similar; see Figure 3. In Plant A, with a 65-ton tapping ladle, the selected steel was pre-deoxidized with C at the beginning of the tapping process and afterwards deoxidized with Al; the slag formers such as lime and MgO were added at the late stage of tapping. In Plant B with a 170-ton ladle, C was added for pre-deoxidation before adding other alloys; MgO was added after the addition of alloy and followed by the addition of other slag formers; all the additions were carried out at the early stage of tapping. The addition schedules of carryover slag as the boundary condition were also defined based on the tapping practice. The mass percentage of the carryover slag components described was assumed to be 30 pct pre-slag, 30 pct vortex slag and 40 pct post slag for Plant A, while for Plant B, the mass percentage was estimated as 5 pct pre-slag, 15 pct vortex slag and 80 pct post slag. The mass of carryover slag was determined based on the mass balance calculation. The compositions of the steel and slag and the temperature before tapping and ladle treatment were measured. To estimate the inclusion content, the total Al and dissolved O concentrations in the steel for Plant A and the total O and dissolved Al concentrations for Plant B were measured.
In the simulations, the adjustable parameters were separately calibrated for each heat by comparing the predictions with the industrial measurements. The recovery rates of C and Mn were adjusted by comparing the corresponding predicted concentrations in the steel with the measured values. The mass transfer coefficient was mainly calibrated by comparing the FeO, MnO and Al2O3 content in the liquid slag. The recovery rate of Al was calibrated by comparing the dissolved O concentration in the steel for the Plant A simulations, while it was adjusted based on the dissolved Al concentration for the Plant B simulations. The inclusion floating rate was adjusted by comparing the total aluminum and oxygen content for the simulations of Plants A and B, respectively. Several simulations were necessary for one heat to achieve the good agreement of the predictions and measurements in the compositions of both the steel and the slag. The air pick-up \( (r_{\text{O}} \;{\text{and}}\;r_{\text{N}} ) \) and addition rate of lining wear (\( r_{\text{lin-st}} \) and \( r_{\text{lin-sl}} \)) were not calibrated in the present simulations and will be considered in future work.
Note that the steel and slag sampling in both plants was carried out before ladle refining. Hence, the steel/slag reactions during transportation and transfer waiting were also considered in the simulations, as shown in Figure 3. In the period of transportation and waiting, the alloying and slag additions that had not dissolved yet dissolved at the same rate as during tapping. During transportation, the mass transfer and inclusion floating were assumed to decrease to one fifth of the values during tapping.
The mean value of the calibrated parameters for the simulations of both Plant A and B are summarized in Table I. Even though the boundary conditions of Plant A and B, such as ladle size, initial compositions of the steel and slag, additions and process operations are different, the mean kinetic parameters of the simulations differ only in reasonable orders. The values of mass transfer coefficient are close to those of intensive stirred ladles.[16,22,23] The comparison of the predicted steel and slag compositions using the mean value listed in Table I and the industrial measurements before LF refining are displayed in Figures 4 and 5.
From Figure 4, it is observed that the predicted and measured steel compositions are in good agreement for the ten heats. Only for one heat, the dissolved Al ([Al]) shows a larger divergence due to an underestimated recovery rate. Figure 4(b) shows that the maximum difference in the predicted total oxygen content, dissolved S ([S]) and P ([P]) concentrations compared with the measurements is approximately 30 ppm, which is believed to be in a reasonable range for the process simulation. As [S] and [P] show a systematic deviation, some room for further improvement exists. Note that so far, all of the components are assumed to have the same mass transfer coefficient at the steel/slag interphase.
In Figure 5, it can be seen that the calculated slag compositions fit well with the measured values. As shown in Figure 5(a), the discrepancies of CaO and Al2O3 content are mostly within 3 pct points and only four comparisons have the discrepancy of 3 to 8 percent points. Figure 5(b) exhibits that the predicted FeO, MnO, SiO2 and MgO content is also in a reasonable range compared to the measured values, which has the largest difference of approximately 5 percent points. Overall, the predicated compositions of the steel and slag with the mean value of the calibrated parameters (Table I) agree well with the measurements, and it is acceptable and appreciated when bearing the complex process and large number of variations in mind.
Results and Discussion
The reasonable agreement of the predictions and measurements discussed in the last section illustrates that the calibrated model is accurate enough to give a general view on the influence of parameter variations in the tapping process, even if it is the clear understanding of the authors that further improvements need to be made in future work. In the following section, one heat from Plant B will be analyzed in detail with respect to the change in the steel composition, slag composition and the development of the inclusion population in time. In a second case study, the amount of carryover slag is varied for the same assumptions in order to investigate the influence of slag carryover on total oxygen and [P] in the steel as well as slag composition before the start of the ladle furnace treatment.
Case Study from Plant B
The predicted composition changes in the steel, slag and inclusions are shown in Figures 6 through 8. For the general tapping and addition schedule, refer to Figure 3. In this case, the tapping time is 6 minutes and the time for transportation amounts to 17 minutes, respectively. Figure 6 displays the concentration development of C, Si, Mn, Al and the total oxygen content in the steel over time. The composition changes during the tapping process (before 360 s) are more significant compared to those during transportation due to the alloying and slag former additions. Once graphite was added at the beginning of tapping, [C] concentration sharply increases (Figure 6(a)) and total oxygen content, as presented in Figure 6(b), decreased correspondingly as a result of CO formation; and then C concentration gradually decreases and total oxygen content increases because of the continuously tapped steel; the amount of CO formation also decreases gradually because of the lower C concentration. The addition and dissolution of Al, MnSi and FeMn at approximately 120 s result in the increases in the concentrations of [C], [Si], [Mn] and [Al]. As shown in Figure 6(b), [Al] concentration quickly drops after a short time of its addition due to inclusion formation and the dilution by tapped steel. Total oxygen content is gradually decreased because of the inclusions floating out, even though the continuously incoming tapped steel offers new dissolved oxygen. When the addition and dissolution of FeMn is completed at approximately 200 s (Figure 6(a)), the concentrations of [C] and [Mn] stop rising. The dilution of the tapped steel leads to a [C] concentration reduction, while the [Mn] concentration changes slightly owing to the continuous addition of MnSi. The [Si] concentration keeps growing until the end of the MnSi addition. Note that there are considerable increases in the concentrations of [Mn] and [Si] after tapping because MnSi dissolution needs time and is only completed during transportation. Afterwards, the concentrations of [C], [Si] and [Mn] barely change, and [Al] concentration and total oxygen content decrease slightly. The predicted steel compositions at the end of the process fit well with the measurements.
Figure 7 displays the predicted slag composition change during tapping and the following transportation process. Figure 8 shows the development of the slag and inclusion phases. At the beginning of tapping, the slag composition is identical to the composition of the BOF slag. In Figure 7(a), the addition and gradual dissolution of MgO in the slag results in an increase in the MgO content and a decrease in the percentages of other components. The MgO content rises even above the saturation limit in the liquid slag, and solid MgO is precipitated, as shown in Figure 8(a). The FeO content (Figure 7(b)) increases before the addition of lime and other slag formers due to the carryover slag. As exhibited in Figure 7(a), the Al2O3 content rises sharply after the addition of Al from 120 to 155 s, and consequently reduces the percentage of other components. The addition of lime and other slag formers (150 s) result in the fast increases in the CaO content. After the end of Al addition (155 s), Al2O3 content in the slag starts to lessen. After the complete dissolution of Al2O3 containing slag formers (230 s), the Al2O3 content falls at a faster speed. CaO content continuously grows until the complete dissolution of lime. The MgO, SiO2 and FeO (Figure 7(b)) content is decreased in the process. From 240 s to 360 s (the end of tapping), there is little change in the MgO, SiO2 and FeO content, which results from the balance of the steel/slag reaction and the continuous addition of carryover slag. The post slag is added by the end of tapping, which leads to a sharp decrease in the Al2O3 (Figure 7(a)) content and an increase in the SiO2 and FeO content (Figure 7(b)); the MgO content remains rather constant and approaches the saturation limit in the liquid slag, illustrated by the continuously increased solid MgO amount (Figure 8(a)). During the transportation, CaO and FeO content decreases slightly due to the steel/slag interfacial reaction, while the Al2O3 content rises.
Figure 8(a) shows that solid CaO and MgO can be stable in the slag besides the liquid solution phase. The slag composition already exceeds the CaO saturation limit at the beginning of tapping, where the slag composition is identical to that of the BOF slag (which is in fact a CaO-saturated slag) and with the increased MgO content (Figure 7(a)). Combining Figures 7(a) and 8(a), the solid MgO phase starts to precipitate with the MgO addition; its amount falls with the growing Al2O3 content and rises with the increasing CaO content; the increase in the amount of solid MgO during transportation is attributed to the temperature dropping by approximately 60 °C. In the refining slag formation process, MgO is added to reach the MgO saturation and protect the lining of the slag line. From the simulation results (Figures 7(a) and 8(a)), MgO addition is sufficient and can even be reduced to some extent to save costs. The liquid slag amount increases during tapping and changes little during the transportation, while the liquid slag formation can be later and more complex in reality.
Figure 8(b) displays the development of the inclusion phases. The three inclusion phases form a liquid phase (slag-liq), CaO·6(Al2O3) and Al2O3. At the beginning of tapping, liquid slag inclusions are formed after graphite addition due to the high C concentration at the interface reducing Si, Ca, Al and Mg from slag to steel. The main composition of the slag-liq phase in this period is 5 pct Al2O3-35 pct SiO2-45 pct CaO-6p ct FeO-4 pct MgO. The content of the slag-liq phase is gradually reduced by floating before the Al addition. After the Al addition, the amount of Al2O3 and slag-liquid grows sharply and the amount of Al2O3 is more than 20 times that of the slag-liquid. The main compositions of the slag-liq phase are 79 pct Al2O3-9 pct CaO-11 pct MgO. A limited amount of CaO·6(Al2O3) also exists. In the following process, most inclusions are removed by floating. Finally, there are approximately 50 ppm of Al2O3 existing together with a negligible amount of CaO·6(Al2O3) which is generated due to the steel/slag reaction before ladle refining.
The predicted and measured compositions of the slag at the start of the ladle furnace treatment correspond very well. The development of the composition seems reasonable. For the inclusion composition, no analytical results are available. Nevertheless, Al2O3 must be the dominating inclusion type and as the predicted total oxygen content fits very well with the measured total oxygen content, the predicted mass fractions must also be reasonable.
Influence of BOF Carryover Slag Amount
Plant B controls slag carryover by applying a slag detection and slag stopper system. The carryover of BOF slag is therefore well controlled. However, the question regarding the influence of a significantly amount deviation carryover slag on the composition of the steel and slag after tapping is of interest. The simulation aims at offering both quality and quantity impressions of the effects of carryover slag, though a comprehensive review of this topic has already been reported.[24] The input parameters are listed in Tables II through IV. Table II gives the composition of the BOF steel, Table III that of the BOF slag and Table IV the assumed input parameters for the performed simulations, not based on industrial data in this case. From Table IV, it is found that three simulations with different carryover slag amounts (2, 6 and 10 kg/ton steel which are typical industrial values[25]) were performed for the Al deoxidation steel, listed in Table I. In the simulations, the assumed tapping and transportation time was assumed to be 5 minutes for each one. The simulation results concerning the influence of carryover slag amount are summarized in Figures 9 through 11.
Figure 9 displays the influence of the carryover slag amount on the liquid slag mass and FeO and MnO content in the liquid slag. As indicated in Figure 9(a), the liquid slag mass increases from 650 to 875 kg and 1100 kg when the carryover slag amount is increased from 130 kg (2 kg/ton) to 390 kg (6 kg/ton) and 650 kg (10 kg/ton). The bigger carryover slag amount directly results in the thicker slag layer and larger interfacial area of the slag and lining. In the meanwhile, the increased carryover slag leads to the increase in FeO and MnO content in the refining slag, as displayed in Figure 9(b), which decreases the slag viscosity and increases the diffusibility of the components[26,27] Hence, more carryover slag enhances the risk of lining corrosion.
From Figure 10(a), it can be seen that Al concentration is reduced with the increasing carryover slag amount. In the simulations, Al as the deoxidizer of steel is further oxidized by the slag through the steel/slag interfacial reaction. As shown in Figure 9, the growing carryover slag leads to the higher slag amount and oxidation ability (FeO and MnO content), which in turn caused the further loss of deoxidizer. The present simulation indicates that approximately 20 kg Al can be saved if the carryover slag amount is reduced from 10 to 2 kg/ton. Figure 10(b) shows that the P reversion rises from 8 to 30 ppm with the carryover slag amount growing from 2 to 10 kg/ton, which is in range of the reported P reversion values.[20,24] It is well known that the slag with high FeO content and basicity supports P holding.[28] In the simulations, the BOF slag contains 1.5 pct P2O5 under the conditions of 27 pct FeO and approximately 4.5 basicity, while the ladle slag before the ladle treatment has an FeO content of 2 to 9 pct (Figure 9(b)) and a basicity of 1.3 to 1.7. Hence, the pick-up of P from the slag is attributed to the lowered P capacity of the slag. Though the FeO content increases with the larger amount of carryover slag, more carryover slag also means more P2O5, leading to an increase in P reversion. Figure 11 displays that the total oxygen content in the steel goes up only slightly under the present assumptions, with the increasing carryover slag amount and slag oxidation ability. In the present simulations, the Al2O3 formed due to Al oxidation in the steel/slag reaction is removed to slag, which can be small particles known as the inclusions generated from reoxidation and remained in the steel. In that case, the total oxygen content increase might be more significant. As a whole, the simulations illustrate the negative effects of carryover slag and the necessity to minimize the amount.
Summary and Outlook
The BOF tapping process was modeled by addressing the chemical reactions. In the modeling process, the thermodynamic calculation was performed using ChemApp. The EERZ method was applied to consider the kinetics of multiphase reactions. The metallurgical actions of the tapping process, such as the addition of alloy and slag former, carryover slag entrapment and inclusion removal, can be considered. The model was calibrated by comparing the predicted steel and slag compositions with industrial input data and measurements. The development of the steel, slag and inclusions during the tapping process was illustrated in detail on the basis of a single heat. The influence of the BOF carryover slag amount was studied using the proposed model. Based on the results, the following conclusions are drawn:
-
The predictions of the steel and slag compositions are in reasonable agreement with the industrial measurements, illustrating the validity of the model.
-
The model can be applied to track the changes in the steel, slag and inclusions during the tapping process.
-
In the simulations with the varied amounts of carryover slag, it was demonstrated that more carryover slag leads to more liquid slag, which increases the interface area of the lining erosion; higher FeO and MnO content in the liquid slag which increases the alloy (Al) consumption and the possibility of reoxidation in the subsequent process; more P reversion from the BOF slag.
In the future, the model will be coupled with a model for the ladle refining process and calibrated in a similar way. The refining process can then be simulated and investigated, addressing the critical reactions and phenomena.
References
1.V. Carreño‐Galindo, R. D. Morales, J. A. Romero, J. F. Chavez and M. V. Toledo: Steel Res. Int., 2000, vol. 71, pp. 107–14.
2.C. Cicutti and C. Capurro: XL CALPHAD, CALPHAD, Rio de Janeiro, Brasil, 2011.
3.M. Tanaka, D. Mazumdar and R. I. L. Guthrie: Metall. Mater. Trans. B, 1993, vol. 24, pp. 639–48.
4.H. Berg, H. Laux and S. T. Johansen: Ironmaking and Steelmaking, 1999, vol. 26, pp. 127–39.
D. You, S. K. Michelic and C. Bernhard: Steel Res. Int., 2020, vol. 91, 2000045.
6.D. You, C. Bernhard, G. Wieser and S. Michelic: Steel Res. Int., 2016, vol. 87, pp. 840–49.
7.D. You, S. K. Michelic, C. Bernhard, D. Loder and G. Wieser: ISIJ Int., 2016, vol. 56, pp. 1770–78.
8.D. You, S. K. Michelic, G. Wieser, and C. Bernhard: J. Mater. Sci., 2016, vol. 52, pp. 1797–812.
9.D. You, S. K. Michelic and C. Bernhard: Metals, 2018, vol. 8, p. 452-69.
10.D. You, S. K. Michelic, C. Bernhard: METEC & 4th ESTAD, The Steel Institute VDEh, Düsseldorf, Germany, 2019, pp. 1–6.
11.S. Petersen and K. Hack: Int. J. Mater. Res., 2007, vol. 98, pp. 935–45.
12.M. A. van Ende, Y. M. Kim, M. K. Cho, J. Choi and I. H. Jung: Metall. Mater. Trans. B, 2011, vol. 42, pp. 477–89.
13.M. A. van Ende and I. H. Jung: Metall. Mater. Trans. B, 2017, vol. 48, pp. 28–36.
14.S. P.T. Piva, D. Kumar and P. C. Pistorius: Metall. Mater. Trans. B, 2016, vol. 48, pp. 37–45.
15.J. H. Shin, Y. Chung and J. H. Park: Metall. Mater. Trans. B, 2017, vol. 48, pp. 46–59.
16.D. Kumar, K. C. Ahlborg and P. C. Pistorius: Metall. Mater. Trans. B, 2019, vol. 50, pp. 2163–74.
17.Y. Ren, L. Zhang and Y. Zhang: J. Iron Steel Res. Int., 2018, vol. 25, pp. 146–56.
18.S. Kitamura, T. Kitamura, K. Shibata, Y. Mizukami, S. Mukawa and J. Nakagawa: ISIJ Int., 1991, vol. 31, pp. 1322–28.
19.B. Enkner, A. Paster and Schwelberger: MPT int., 2001, vol. 24, pp.40-42.
G. Schnalzger, P. Bundschuh, R. Rössler: J. Schenk and A. Viertauer: RHI Magnesita Worldwide, 2017, pp.76–81.
21.A. Harada, N. Maruoka, H. Shibata and S. Kitamura: ISIJ Int., 2013, vol. 53, pp. 2110–17.
J. Peter, K. D. Peaslee, D. G. C. Robertson, M. Hall and B. G. Thomas: Proc. AISTech 2005, Vol. 1, Association for Iron & Steel Technology, Warrendale, 2005, pp. 959–73.
K. J. Graham and G. A. Irons, Proc. AISTech 2009, Vol. 1, Association for Iron & Steel Technology, Warrendale, 2009, pp. 1003–14.
24.P. C. Pistorius: J. S. Afr. Inst. Min. Metall., 2019, vol. 119, pp. 557-561.
G. Böcher, U. Grethe, J. Kempken, E. Schnurrenberger and H. Müller: 3rd European Oxygen Steelmaking Conference, Birmingham, UK, 2000, pp. 1–9.
26.S. Lee and D. J. Min: Steel Res. Int., 2018, vol. 89, 1800055.
27.M. Lee, S. Sun, S. Wright and S. Jahanshahi: Metall. Mater. Trans. B, 2001, vol. 32, pp. 25–29.
28.S. Basu, A. K. Lahiri and S. Seetharaman: Metall. Mater. Trans. B, 2007, vol. 38, pp. 357–66.
Acknowledgments
The authors gratefully acknowledge the funding support of K1-MET GmbH, metallurgical competence centre. The research program of the competence centre K1-MET is supported by COMET (Competence Centre for Excellent Technologies), the Austrian program for competence centres. COMET is funded by the Federal Ministry for Transport, Innovation and Technology, the Federal Ministry for Digital and Economic Affairs, the province of Upper Austria, Tyrol, and Styria, the Styrian Business Promotion Agency. A partial financing comes from scientific and the industrial partners: voestalpine Stahl, voestalpine Stahl Donawitz, Primetals Technologies Austria and RHI Magnesita.
Funding
Open access funding provided by Montanuniversität Leoben.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted January 27, 2021; accepted March 11, 2021.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
You, D., Bernhard, C., Mayer, P. et al. Modeling of the BOF Tapping Process: The Reactions in the Ladle. Metall Mater Trans B 52, 1854–1865 (2021). https://doi.org/10.1007/s11663-021-02153-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-021-02153-2