Abstract
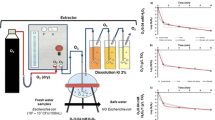
The successful use of ozonation for water disinfection and purification requires the determination of optimal treatment conditions, which is associated with a large amount of experiments. At the same time, traditional methods for controlling the process of inactivation of microorganisms, being quite laborious and time consuming, allow one to obtain data on the degree of water disinfection only after 18–48 h after the experiment and sampling. Given that ozone is an oxidizing reagent and its presence in the treated water causes a change in the oxidation-reduction potential (ORP) of the solution, this study is aimed at changing the ORP during ozone disinfection of water and at investigating the possibility of express control of this process. As objects of study, we have used model solutions containing 104–107 CFU/cm3 of Escherichia coli and various concentrations (0–20 mg/dm3) of humic acids (HA) in a 0.05 M phosphate buffer at pH 6.8, as well as real natural waters of the Dnieper and Desna Rivers. The ozone concentration in the ozone–air mixture varies in the range of 0.5–2.0 mg/dm3, depending on the particular object of study. It is shown that changes in the ORP of a solution reflect changes in the microbiological and chemical composition of water during ozonation, owing to which measurements of this indicator can serve as a preliminary control method for the process of disinfection, as well as water decoloration. The inflection point on the curve of dependence of ORP on ozonation time practically coincides with the inflection point on the curve of dependence of the degree of water disinfection on the treatment time. In the case of treatment of model waters that are free of organic impurities, a sufficient level of disinfection of the solution is evidenced by an increase in the ORP of the solution to about 440–550 mV. In case of treating natural surface waters (with a high content of natural organic impurities), a sufficient level of disinfection and decoloration of the solution is detected by a decrease in the ORP of the solution by about 30–40 mV.


Similar content being viewed by others
REFERENCES
Wei, C., Zhang, F., Hu, Y., Feng, C., and Wu, H., Ozonation in water treatment: the generation, basic properties of ozone and its practical application, Rev. Chem. Eng., 2017, vol. 33, no. 1, pp. 49–89. https://doi.org/10.1515/revce-2016-0008
Loeb, B.L., Thompson, C.M., Drago, J., Takahara, H., and Baig, S., Worldwide ozone capacity for treatment of drinking water and wastewater: A review, Ozone: Sci. Eng., 2012, vol. 34, no. 1, pp. 64–77. https://doi.org/10.1080/01919512.2012.640251
Xiao, J., Xie, Y., and Cao, H., Organic pollutants removal in wastewater by heterogeneous photocatalytic ozonation, Chemosphere, 2015, vol. 121, pp. 1–17. https://doi.org/10.1016/j.chemosphere.2014.10.072
De Vera, G.A., Stalter, D., Gernjak, W., Weinberg, H.S., Keller, J., and Farré, M.J., Towards reducing DBP formation potential of drinking water by favoring direct ozone over hydroxyl radical reactions during ozonation, Water Res., 2015, vol. 87, pp. 49–58. https://doi.org/10.1016/j.watres.2015.09.007
Liu, C., Tang, X., Kim, J., and Korshin, G.V., Formation of aldehydes and carboxylic acids in ozonated surface water and wastewater: A clear relationship with fluorescence changes, Chemosphere, 2015, vol. 125, pp. 182–190. https://doi.org/10.1016/j.chemosphere.2014.12.054
Rojas-Serrano, F., Pérez, J.I., and Gómez, M. Á., Comparative study of in-line coagulation and/or ozonization pre-treatment for drinking-water production with spiral-wound ultrafiltration membranes, Chem. Eng. Process., 2016, vol. 105, pp. 21–29. https://doi.org/10.1016/j.cep.2016.04.004
Lüddeke, F., Heß, S., Gallert, C., Winter, J., Güde, H., and Löffler, H., Removal of total and antibiotic resistant bacteria in advanced wastewater treatment by ozonation in combination with different filtering techniques, Water Res., 2015, vol. 69, pp. 243–251. https://doi.org/10.1016/j.watres.2014.11.018
Miao, H. and Tao, W., Ozonation of humic acid in water, J. Chem. Technol. Biotechnol., 2008, vol. 8, no. 3, pp. 336–344. https://doi.org/10.1002/jctb.1816
MV 10.2.1-113-2005: Sanitary and Microbiological Control of Drinking Water Quality, Kyiv, 2005, no. 60.
Saprykina, M.N., Bolgova, E.V., Mel’nik, L.A., and Goncharuk, V.V., The effect of physicochemical parameters on the process of water disinfection using chitosan, J. Water Chem. Technol., 2019, vol. 41, no. 6, pp. 384–390. https://doi.org/10.3103/s1063455x19060079
Goncharuk, V.V., Potapchenko, N.G., Savluk, O.S., Kosinova, V.N., and Sova, A.N., Disinfection of water by ozone: Effect of inorganic impurities on kinetics of water disinfection, Khim. Tekhnol. Vody, 2001, vol. 23, no. 2, pp. 55–63.
Goncharuk, V.V., Bagrii, V.A., Mel’nik, L.A., Chebotareva, R.D., and Bashtan, S.Yu., The use of redox potential in water treatment processes, J. Water Chem. Technol., 2010, vol. 32, no. 1, pp. 1–9. https://doi.org/10.3103/s1063455x10010017
Yu, R.-F., Chen, H.-W., Cheng, W.-P., and Shen, Y.-C., Dynamic control of disinfection for wastewater reuse applying ORP/pH monitoring and artificial neural networks, Resour., Conserv. Recycl., 2008, vol. 52, nos. 8–9, pp. 1015–1021. https://doi.org/10.1016/j.resconrec.2008.03.007
Bastian, T. and Brondum, J., Do traditional measures of water quality in swimming pools and spas correspond with beneficial oxidation reduction potential? Public Health Rep., 2009, vol. 124, no. 2, pp. 255–261. https://doi.org/10.1177/003335490912400213
Suslow, T.V., Oxidation-Reduction Potential (ORP) for Water Disinfection Monitoring, Control, and Documentation, San Diego: Univ. of Calif., 2004. https://doi.org/10.3733/ucanr.8149
Kerc, A., Bekbolet, M., and Seatci, A.M., Effects of oxidative treatment techniques on moleculare size distribution of humic acids, J. Water Sci. Technol., 2004, vol. 49, no. 4, pp. 7–12. https://doi.org/10.2166/wst.2004.0205
Klimenko, N.A., Samsoni-Todorova, E.A., Savchina, L.A., Lavrenchuk, I.N., and Zasyad’ko, T.N., Seasonal variations of characteristics of organic matter in the Dnieper River water, J. Water Chem. Technol., 2012, vol. 34, no. 3, pp. 154–161. https://doi.org/10.3103/s1063455x1203006x
Goncharuk, V.V., Vakulenko, V.F., Sova, A.N., Oleynik, L.M., and Shvadchina, Yu.O., Effect of UV irradiation modes on the kinetics and degradation degree of humic and fulvic acids by ozone, J. Water Sci. Technol., 2003, vol. 25, no. 5, pp. 1–17.
DSTU 525-2014: Drinking Water. Quality Control Requirements and Methods, Kyiv: Minist. Ekon. Rozvit. Ukr., 2014.
Milyukin, M.V., Vakulenko, V.F., and Goncharuk, V.V., Sostav karbonil`nykh soedinenij pri ozonirovanii i O3/UF-obrabotke vody, Ukr. Khim. Zh., 2007, vol. 73, no. 3, pp. 48–55.
Rogov, V.M. and Filipchuk, V.L., Elektrokhimicheskaya tekhnologiya izmeneniya svoistv vody (Electrochemical Change of Water Properties), L’vov: L'vov. Gos. Univ., 1989.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Kadkin
About this article
Cite this article
Melnik, L.O., Vakulenko, V.F., Saprykina, M.M. et al. Change of the Oxidation-Reduction Potential of Model and Natural Waters in the Ozone Disinfection Process. J. Water Chem. Technol. 43, 85–91 (2021). https://doi.org/10.3103/S1063455X21010094
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1063455X21010094