Abstract
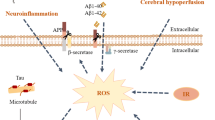
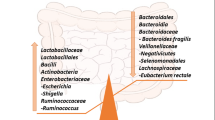
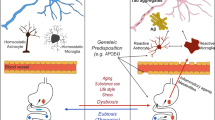
Alzheimer’s disease (AD) is a neurodegenerative disorder, and its pathogenesis is not fully known. Although there are several hypotheses, such as neuroinflammation, tau hyperphosphorylation, amyloid-β plaques, neurofibrillary tangles, and oxidative stress, none of them completely explain the origin and progression of AD. Emerging evidence suggests that gut microbiota and epigenetics can directly influence the pathogenesis of AD via their effects on multiple pathways, including neuroinflammation, oxidative stress, and amyloid protein. Various gut microbes such as Actinobacteria, Bacteroidetes, E. coli, Firmicutes, Proteobacteria, Tenericutes, and Verrucomicrobia are known to play a crucial role in the pathogenesis of AD. These microbes and their metabolites modulate various physiological processes that contribute to AD pathogenesis, such as neuroinflammation and other inflammatory processes, amyloid deposition, cytokine storm syndrome, altered BDNF and NMDA signaling, impairing neurodevelopmental processes. Likewise, epigenetic markers associated with AD mainly include histone modifications and DNA methylation, which are under the direct control of a variety of enzymes, such as acetylases and methylases. The activity of these enzymes is dependent upon the metabolites generated by the host’s gut microbiome, suggesting the significance of epigenetics in AD pathogenesis. It is interesting to know that both gut microbiota and epigenetics are dynamic processes and show a high degree of variation according to diet, stressors, and environmental factors. The bidirectional relation between the gut microbiota and epigenetics suggests that they might work in synchrony to modulate AD representation, its pathogenesis, and progression. They both also provide numerous targets for early diagnostic biomarkers and for the development of AD therapeutics. This review discusses the gut microbiota and epigenetics connection in the pathogenesis of AD and aims to highlight vast opportunities for diagnosis and therapeutics of AD.



Similar content being viewed by others
References
Abreu MT (2010) Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol 10(2):131–144
Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR et al (2011) Enterotypes of the human gut microbiome. Nature 473(7346):174–180
Askarova S, Umbayev B, Masoud AR, Kaiyrlykyzy A, Safarova Y, Tsoy A et al (2020) The links between the gut microbiome, aging, modern lifestyle and Alzheimer’s Disease. Front Cell Infect Microbiol 10:104
Asti A, Gioglio L (2014) Can a bacterial endotoxin be a key factor in the kinetics of amyloid fibril formation? J Alzheimers Dis 39(1):169–179
Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM et al (2010) Early life programming and neurodevelopmental disorders. Biol Psychiatry 68:314–319
Barker DJP (1997) Maternal nutrition, fetal nutrition, and disease in later life. Nutrition 13:807–813
Barnham KJ, Masters CL, Bush AI (2004) Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov 3:205–214
Bassett CN, Montine TJ (2003) Lipoproteins and lipid peroxidation in Alzheimer’s disease. J Nutr Health Aging 7:24–29
Bauer H, Horowitz RE, Levenson SM, Popper H (1963) The response of the lymphatic tissue to the microbial flora. Studies on germfree mice. Am J Pathol 42(4):471
Becker KG (2007) Autism, asthma, inflammation, and the hygiene hypothesis. Med Hypotheses 69:731–740
Belkaid Y, Hand TW (2014) Role of the microbiota in immunity and inflammation. Cell 157(1):121–141
Berr C (2000) Cognitive impairment and oxidative stress in the elderly: results of epidemiological studies. BioFactors 13:205–209
Bhattacharjee S, Lukiw WJ (2013) Alzheimer’s disease and the microbiome. Front Cell Neurosci 7:153
Bilbo SD, Levkoff LH, Mahoney JH, Watkins LR, Rudy JW, Maier SF (2005) Neonatal infection induces memory impairments following an immune challenge in adulthood. Behav Neurosci 119:293–301
Bolduc JF, Hany L, Barat C, Ouellet M, Tremblaya MJ (2017) Epigenetic metabolite acetate inhibits class I/II histone deacetylases, promotes histone acetylation, and increases HIV-1 Integration in CD4 T cells. J Virol 91:1–15
Bonfili L, Cecarini V, Berardi S, Scarpona S, Suchodolski JS, Nasuti C et al (2017) Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci Rep 7(1):1–21
Bonnin A, Goeden N, Chen K, Wilson ML, King J, Shih JC et al (2011) A transient placental source of serotonin for the fetal forebrain. Nature 472:347–350
Borrego S, Vazquez A, Dasí F, Cerdá C, Iradi A, Tormos C et al (2013) Oxidative stress and DNA damage in human gastric carcinoma: 8-Oxo-7’8-dihydro-2’-deoxyguanosine (8-oxo-dG) as a possible tumor marker. Int J Mol Sci 14(2):3467–3486
Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Toth M, et al (2014) The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med 6:263ra158
Buffie CG, Pamer EG (2013) Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol 13(11):790–801
Bungard D, Fuerth BJ, Zeng PY, Faubert B, Maas NL, Viollet B et al (2010) Signaling kinase AMPK activates stress-promoted transcription via histone H2B phosphorylation. Science 329(5996):1201–1205
Butovsky O, Kunis G, Koronyo-Hamaoui M, Schwartz M (2007) Selective ablation of bone marrow-derived dendritic cells increases amyloid plaques in a mouse Alzheimer’s disease model. Eur J Neurosci 26(2):413–416
Campos PB, Paulsen BS, Rehen SK (2014) Accelerating neuronal aging in in-vitro model brain disorders: A focus on reactive oxygen species. Front Aging Neurosci 6:292
Carabotti M, Scirocco A, Maselli MA, Severi C (2015) The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 28(2):203
Carbonero F (2017) Human epigenetics and microbiome: the potential for a revolution in both research areas by integrative studies
Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ (2015) Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis 26(1):26191
Cattaneo A, Cattane N, Galluzzi S, Provasi S, Lopizzo N, Festari C et al (2017) Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging 49:60–68
Chen SG, Stribinskis V, Rane MJ, Demuth DR, Gozal E, Roberts AM et al (2016) Exposure to the functional bacterial amyloid protein curli enhances alpha-synuclein aggregation in aged Fischer 344 rats and Caenorhabditis elegans. Sci Rep 6(1):1
Chouliaras L, Rutten BP, Kenis G, Peerbooms O, Visser PJ, Verhey F et al (2010) Epigenetic regulation in the pathophysiology of Alzheimer’s disease. Progress Neurobiol 90(4):498–510
Christen Y (2000) Oxidative stress and Alzheimer disease. Am J Clin Nutr 71:621–629
Clapier CR, Cairns BR (2009) The biology of chromatin remodeling complexes. Annu Rev Biochem 7(8):273–304
Clemente JC, Ursell LK, Parfrey LW, Knight R (2012) The impact of the gut microbiota on human health: an integrative view. Cell 148(6):1258–1270
Cong X, Xu W, Janton S, Henderson WA, Matson A, McGrath JM, et al (2016) Gut microbiome developmental patterns in early life of preterm infants: impacts of feeding and gender. PloS One 11(4)
Coppedè F (2014) The potential of epigenetic therapies in neurodegenerative diseases. Front Genet 5:220
Crabbe PA, Bazin H, Eyssen H, Heremans JF (1968) The normal microbial flora as a major stimulus for proliferation of plasma cells synthesizing IgA in the gut. Int Arch Allergy Immunol 34(4):362–375
Cronk JC, Filiano AJ, Louveau A, Marin I, Marsh R, Ji E, Goldman DH, Smirnov I, Geraci N, Acton S, Overall CC (2018) Peripherally derived macrophages can engraft the brain independent of irradiation and maintain an identity distinct from microglia. J Exp Med 215(6):1627–1647
Cryan JF, Dinan TG (2012) Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 13(10):701–712
Dawson TM, Dawson VL, Snyder SH (1992) A novel neuronal messenger molecule in brain: the free radical, nitric oxide. Ann Neurol 32(3):297–311
Day JJ, Sweatt JD (2010) DNA methylation and memory formation. Nat Neurosci 13(11):1319–1323
Delgado-Morales R, Esteller M (2017) Opening up the DNA methylome of dementia. Mol Psychiatry 22(4):485–496
Den H, Dong X, Chen M, Zou Z (2020) Efficacy of probiotics on cognition, and biomarkers of inflammation and oxidative stress in adults with Alzheimer’s disease or mild cognitive impairment—a meta-analysis of randomized controlled trials. Aging (Albany NY) 12(4):4010
Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A et al (2011) Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci 108:3047–3052
Dickson DW, Lee SC, Mattiace LA, Yen S-HC, Brosnan C (1993) Microglia and cytokines in neurological disease, with special reference to AIDS and Alzheimer’s disease Glia 7:75–83
Dicksved J, Schreiber O, Willing B, Petersson J, Rang S, Phillipson M, Holm L, Roos S (2012) Lactobacillus reuteri maintains a functional mucosal barrier during DSS treatment despite mucus layer dysfunction. PLoS One 7(9):e46399
Dror DK, Allen LH (2008) Effect of vitamin B12 deficiency on neurodevelopment in infants: current knowledge and possible mechanisms. Nutr Rev 66:250–255
Dumitrescu L, Popescu-Olaru I, Cozma L, Tulbă D, Hinescu ME, Ceafalan LC, et al (2018) Oxidative stress and the microbiota-gut-brain axis. Oxid Med Cellular Longev 2018
Durany N, Münch G, Michel T, Riederer P (1999) Investigations on oxidative stress and therapeutical implications in dementia. Eur Arch Psychiatry Clin Neurosci 249:68–73
Emery DC, Shoemark DK, Batstone TE et al (2017) 16S rRNA next generation sequencing analysis shows bacteria in Alzheimer’s post-mortem brain. Front Aging Neurosci 9:195
Erny D, de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, Schwierzeck V (2015) Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 18(7):965–977
Ertekin-Taner N (2007) Genetics of Alzheimer’s disease: A centennial review. Neurol Clin 25(3):611–667
Esposito M, Sherr GL (2019) Epigenetic modifications in Alzheimer’s neuropathology and therapeutics. Front Neurosci 13:476
Fakhoury M (2018) Microglia and astrocytes in Alzheimer’s disease: Implications for therapy. Current Neuropharmacol 16(5):508–518
Farhadi A, Banan AL, Fields J, Keshavarzian AL (2003) Intestinal barrier: an interface between health and disease. J Gastroenterol Hepatol 18(5):479–497
Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen ML, Bolte E et al (2002) Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis 35(Suppl 1):S6-16
Finegold SM (2008) Therapy and epidemiology of autism clostridial spores as key elements. Med Hypotheses 70:508–511
Forster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS (1996) Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc Natl Acad Sci USA 93:4765–4769
Fox M, Knorr DA, Haptonstall KM (2019) Alzheimer’s disease and symbiotic microbiota: an evolutionary medicine perspective. Ann N Y Acad Sci 1449:3–24
Francis YI, Fa M, Ashraf H, Zhang H, Staniszewski A, Latchman DS et al (2009) Dysregulation of histone acetylation in the APP/PS1 mouse model of Alzheimer’s disease. J Alz Dis 18(1):131–139
Fransen F, van Beek AA, Borghuis T, Aidy SE, Hugenholtz F, van der Gaast-de JC et al (2017) Aged gut microbiota contributes to systemical inflammaging after transfer to germ-free mice. Front Immunol 8:1385
Friedland RP, Chapman MR (2017) The role of microbial amyloid in neurodegeneration. PLoS Pathogens 13(12)
Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA et al (2018) Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front Immunol 8:1960
Gaboriau-Routhiau V, Rakotobe S, Lécuyer E, Mulder I, Lan A, Bridonneau C, Rochet V, Pisi A, De Paepe M, Brandi G, Eberl G (2009) The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 31(4):677–689
Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ et al (2011) Bacterial infection causes stress-induced memory dysfunction in mice. Gut 60:307–317
Gareau MG, Silva MA, Perdue MH (2008) Pathophysiological mechanisms of stress-induced intestinal damage. Curr Mol Med 8:274–281
Garzon D, Yu G, Fahnestock M (2002) A new brain-derived neurotrophic factor transcript and decrease inbrain-derived neurotrophic factor transcripts 1, 2 and 3 in Alzheimer’s disease parietal cortex. J Neurochem 82(5):1058–1064
Gill N, Wlodarska M, Finlay BB (2011) Roadblocks in the gut: barriers to enteric infection. Cell Microbiol 13(5):660–669
Goehler LE, Park SM, Opitz N, Lyte M, Gaykema RP (2008) Campylobacter jejuni infection increases anxietylike behavior in the holeboard: Possible anatomical substrates for viscerosensory modulation of exploratory behavior. Brain Behav Immun 22:354–366
Goussakov I, Miller MB, Stutzmann GE (2010) NMDA-mediated Ca2+ influx drives aberrant ryanodine receptor activation in dendrites of young Alzheimer’s disease mice. J Neurosci 30(36):12128–12137
Gräff J, Rei D, Guan JS, Wang WY, Seo J, Hennig KM et al (2012) An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature 483(7388):222–226
Griñán-Ferré C, Corpas R, Puigoriol-Illamola D, Palomera-Ávalos V, Sanfeliu C, Pallàs M (2018) Understanding epigenetics in the neurodegeneration of Alzheimer’s disease: SAMP8 mouse model. J Alzheimers Dis 62(3):943–963
Greenblum S, Turnbaugh PJ, Borenstein E (2012) Metagenomic systems biology of the human gut microbiome reveals topological shifts associated with obesity and inflammatory bowel disease. Proc Natl Acad Sci 109(2):594–599
Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ et al (1989) Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci USA 86:7611–7615
Guxens M, Mendez MA, Moltó-Puigmartí C, Julvez J, García-Esteban R, Forns J et al (2011) Breastfeeding, longchain polyunsaturated fatty acids in colostrum, and infant mental development. Pediatrics 128:e880–e889
Hammond CJ, Hallock LR, Howanski R, Appelt DM, Little CS, Balin BJ (2010) Immunohistological detection of Chlamydia pneumoniae in the Alzheimer's disease brain. BMC Neuroscience 11(1)
Harach T, Marungruang N, Duthilleul N, Cheatham V, Mc Coy KD, Frisoni G et al (2017) Reduction of Ab amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci Rep 7:46856
Haverson K, Rehakova Z, Sinkora J, Sver L, Bailey M (2007) Immune development in jejunal mucosa after colonization with selected commensal gut bacteria: a study in germ-free pigs. Vet Immunol Immunopathol 119(3–4):243–253
Heneka MT, Carson MJ, Khoury JE, Landreth GE, Brosseron F, Feinstein DL et al (2015) Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14:388–405
Henry C, Kabbaj M, Simon H, Le Moal M, Maccari S (1994) Prenatal stress increases the hypothalamo-pituitary-adrenal axis response in young and adult rats. J Neuroendocrinol 6:341–345
Hesson LB (2013) Gut microbiota and obesity-related gastrointestinal cancer: a focus on epigenetics. Trans Gastrointest Cancer 2(4):204–210
Hill DA, Hoffmann C, Abt MC, Du Y, Kobuley D, Kirn TJ, Bushman FD, Artis D (2010) Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal Immunol 3(2):148–158
Hill DR, Huang S, Nagy MS, Yadagiri VK, Fields C, Mukherjee D et al (2017) Bacterial colonization stimulates a complex physiological response in the immature human intestinal epithelium. Elife 6:e29132
Ho L, Ono K, Tsuji M, Mazzola P, Singh R, Pasinetti GM (2018) Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer’s disease-type beta-amyloid neuropathological mechanisms. Expert Rev Neurother 18(1):83–90
Hoeijmakers L, Heinen Y, Van Dam AM, Lucassen PJ, Korosi A (2016) Microglial priming and Alzheimer’s disease: a possible role for (early) immune challenges and epigenetics? Front Human Neurosci 10:398
Holzer P, Farzi A (2014) Neuropeptides and the microbiota-gut-brain axis. InMicrobial endocrinology: The microbiota-gut-brain axis in health and disease (pp. 195–219). Springer, New York, NY
Holzer P, Danzer M, Schicho R, Samberger C, Painsipp E, Lippe IT (2004) Vagal afferent input from the acid-challenged rat stomach to the brainstem: enhancement by interleukin-1β. Neuroscience 129(2):439–445
Houghteling PD, Walker WA (2015) Why is initial bacterial colonization of the intestine important to the infant’s and child’s health? J Pediatr Gastroenterol Nutr 60(3):294
Hu N, Tan MS, Yu JT, Sun L, Tan L, Wang YL, Jiang T, Tan L (2014) Increased expression of TREM2 in peripheral blood of Alzheimer’s disease patients. J Alzheimers Dis 38(3):497–501
Hughes HK, Rose D, Ashwood P (2018) The gut microbiota and dysbiosis in autism spectrum disorders. Curr Neurol Neurosci Rep 18(11):81
Itzhaki RF, Wozniak MA, Appelt DM, Balin BJ (2004) Infiltration of the brain by pathogens causes Alzheimer’s disease. Neurobiol Aging 25:619–627
Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T (2009) Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139(3):485–498
Ivashko-Pachima Y, Hadar A, Grigg I, Korenková V, Kapitansky O, Karmon G, Gershovits M, Sayas CL, Kooy RF, Attems J, Gurwitz D (2019) Discovery of autism/intellectual disability somatic mutations in Alzheimer's brains: mutated ADNP cytoskeletal impairments and repair as a case study. Molecular psychiatry 1–5
Jiang C, Li G, Huang P, Liu Z, Zhao B (2017) The gut microbiota and Alzheimer’s disease. J Alzheimers Dis 58(1):1–5
Kaelberer MM, Buchanan KL, Klein ME, Barth BB, Montoya MM, Shen X, et al (2018) A gut-brain neural circuit for nutrient sensory transduction. Science 361(6408):eaat5236.
Kamada N, Seo SU, Chen GY, Núñez G (2013) Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol 13(5):321–335
Katada S, Imhof A, Sassone-Corsi P (2012) Connecting threads: epigenetics and metabolism. Cell 148(1–2):24–28
Kietzmann T, Petry A, Shvetsova A, Gerhold JM, Görlach A (2017) The epigenetic landscape related to reactive oxygen species formation in the cardiovascular system. Br J Pharmacol 174(12):1533–1554
Kim VN (2006) Small RNAs just got bigger: Piwi-interacting RNAs (piRNAs) in mammalian testes. Genes Dev 20(15):1993–1997
Kim YK, Shin C (2018) The microbiota-gut-brain axis in neuropsychiatric disorders: pathophysiological mechanisms and novel treatments. Curr Neuropharmacol 16(5):559–573
Kodis EJ, Choi S, Swanson E, Ferreira G, Bloom GS (2018) N-methyl-D-aspartate receptor–mediated calcium influx connects amyloid-β oligomers to ectopic neuronal cell cycle reentry in Alzheimer’s disease. Alzheimers Dement 14(10):1302–1312
Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F (2016) From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165(6):1332–1345
Kondo Y (2009) Epigenetic cross-talk between DNA methylation and histone modifications in human cancers. Yonsei Med J 50(4):455–463
Kountouras J, Tsolaki M, Gavalas E et al (2006) Relationship between Helicobacter pylori infection and Alzheimer disease. Neurology 66:938–940
Kowalski K, Mulak A (2019) Brain-gut-microbiota axis in Alzheimer’s disease. J Neurogastroenterol Motil 25:48–60
Krabbe G, Halle A, Matyash V, Rinnenthal JL, Eom GD, Bernhardt U et al (2013) Functional impairment of microglia coincides with b-amyloid deposition in mice with Alzheimer-like pathology. PLoS One 8:e60921
Kramer MS, Aboud F, Mironova E, Vanilovich I, Platt RW, Matush L et al (2008) Promotion of Breastfeeding Intervention Trial (PROBIT) Study Group. Breastfeeding and child cognitive development: new evidence from a large randomized trial. Arch Gen Psychiatry 65:578–84
Krautkramer KA, Dhillon RS, Denu JM, Carey HV (2017) Metabolic programming of the epigenome: host and gut microbial metabolite interactions with host chromatin. Transl Res 189:30–50
Kumar H, Lund R, Laiho A, Lundelin K, Ley RE, Isolauri E et al (2014) Gut microbiota as an epigenetic regulator: pilot study based on whole-genome methylation analysis. MBio 5(6):e02113-e2114
Leclercq S, Mian FM, Stanisz AM, Bindels LB, Cambier E, Ben-Amram H et al (2017) Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat Commun 8(1):1–2
Lee JW, Lee YK, Yuk DY, Choi DY, Ban SB, Oh KW et al (2008) Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J Neuroinflamm 5:37
Li Z, Zhu H, Zhang L, Qin C (2018) The intestinal microbiome and Alzheimer’s disease: A review. Animal Model Exp Med 1(3):180–188
Liévin-Le Moal V, Servin AL (2006) The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: mucins, antimicrobial peptides, and microbiota. Clin Microbiol Rev 19:315–337
Lin L, Zheng LJ, Zhang LJ (2018) Neuroinflammation, gut microbiome and Alzheimer’s disease. Mol Neurobiol 55:8243–8250
Liu XS, Wu H, Ji X, Stelzer Y, Wu X, Czauderna S et al (2016) Editing DNA methylation in the mammalian genome. Cell 167(1):233–247
Liu P, Wu L, Peng G, Han Y, Tang R, Ge J, Zhang L, Jia L, Yue S, Zhou K, Li L (2019) Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav Immun 80:633–643
Logsdon AF, Erickson MA, Rhea EM, Salameh TS, Banks WA (2018) Gut reactions: How the blood–brain barrier connects the microbiome and the brain. Exp Biol Med 243(2):159–165
Lovell MA, Markesbery WR (2007) Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer’s disease. Nucleic Acids Res 35:7497–7504
Lu B (2003 BDNF and activity-dependent synaptic modulation. Learn Mem 10:86–98
Luczynski P, Whelan SO, O’Sullivan C, Clarke G, Shanahan F, Dinan TG et al (2016) Adult microbiota-deficient mice have distinct dendritic morphological changes: differential effects in the amygdala and hippocampus. Eur J Neurosci 44:2654–2666
Lukiw WJ (2016) Bacteroides fragilis lipopolysaccharide and inflammatory signaling in Alzheimer’s disease. Front Microbiol 7:1544
Lyte M (2013) Microbial endocrinology in the microbiome-gut-brain axis: how bacterial production and utilization of neurochemicals influence behavior. PLoS Pathog 9(11):e1003726
Macpherson AJ, Martinic MM, Harris N (2002) The functions of mucosal T cells in containing the indigenous commensal flora of the intestine. Cellular and Molecular Life Sciences CMLS 59(12):2088–2096
Maes M (2008) The cytokine hypothesis of depression: inflammation, oxidative & nitrosative stress (IO&NS) and leaky gut as new targets for adjunctive treatments in depression. Neuro Endocrinol Lett 29:287–291
Mani V (2012) Understanding intestinal lipopolysaccharide permeability and associated inflammation
Marques TM, Wall R, Ross RP, Fitzgerald GF, Ryan CA, Stanton C (2010) Programming infant gut microbiota: influence of dietary and environmental factors. Curr Opin Biotechnol 21:149–156
Martin CR, Osadchiy V, Kalani A, Mayer EA (2018) The brain-gut-microbiome axis. Cell Mol Gastroenterol Hepatol 6(2):133–148
Maslowski KM, Mackay CR (2011) Diet, gut microbiota and immune responses. Nat Immunol 12(1):5–9
Mayer EA, Savidge T, Shulman RJ (2014) Brain-gut microbiome interactions and functional bowel disorders. Gastroenterol 146:1500–1512
Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL (2005) An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122(1):107–118
McCormick CM, Smythe JW, Sharma S, Meaney MJ (1995) Sex-specific effects of prenatal stress on hypothalamicpituitary-adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Brain Res Dev Brain Res 84:55–61
McGeer PL, McGeer EG (2002) Local neuroinflammation and the progression of Alzheimer’s disease. J Neurovirol 8:529–538
McVey Neufeld KA, Kay S, Bienenstock J (2018) Mouse strain affects behavioral and neuroendocrine stress responses following administration of probiotic Lactobacillus rhamnosus JB-1 or traditional antidepressant fluoxetine. Front Neurosci 12:294
Mehta V, Parashar A, Sharma A, Singh TR, Udayabanu M (2017a) Quercetin ameliorates chronic unpredicted stress-mediated memory dysfunction in male Swiss albino mice by attenuating insulin resistance and elevating hippocampal GLUT4 levels independent of insulin receptor expression. Horm Behav 89:13–22
Mehta V, Parashar A, Udayabanu M (2017b) Quercetin prevents chronic unpredictable stress induced behavioral dysfunction in mice by alleviating hippocampal oxidative and inflammatory stress. Physiol Behav 171:69–78
Menard S, Cerf-Bensussan N, Heyman M (2010) Multiple facets of intestinal permeability and epithelial handling of dietary antigens. Mucosal Immunol 3(3):247–259
Michalski B, Fahnestock M (2003) Pro-brain-derived neurotrophic factor is decreased in parietal cortex in Alzheimer’s disease. Mol Brain Res 111(1–2):148–154
Mikkelsen HB, Garbarsch C, Tranum-Jensen J, Thuneberg L (2004) Macrophages in the small intestinal muscularis externa of embryos, newborn and adult germ-free mice. J Mol Histol 35(4):377–387
Millan MJ (2014) The epigenetic dimension of Alzheimer’s disease: causal, consequence, or curiosity? Dialogues Clin Neurosci 16(3):373
Minter MR, Zhang C, Leone V, Ringus DL, Zhang X, Oyler-Castrillo P et al (2016) Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci Rep 6:30028
Mir FA, Rizvi ZA (2019) Neurobiological mechanisms involved in the pathogenesis of Alzheimer’s disease. In Biological, Diagnostic and Therapeutic Advances in Alzheimer's Disease (pp. 235–269)
Mittal VA, Ellman LM, Cannon TD (2008) Gene environment interaction and covariation in schizophrenia: the role of obstetric complications. Schizophr Bull 34:1083–1094
Mohsen MMAE, Iravani MM, Spencer JPE, Rose S, Fahim AT, Motawi TMK et al (2005) Ageassociated changes in protein oxidation and proteasome activities in rat brain: Modulation by anti-oxidants. Biochemical Biophysical Res Commun 336:386–391
Mori Y, Yoshino Y, Ochi S, Yamazaki K, Kawabe K, Abe M, Kitano T, Ozaki Y, Yoshida T, Numata S, Mori T (2015) TREM2 mRNA expression in leukocytes is increased in Alzheimer’s disease and schizophrenia. PLoS One 10(9):e0136835
Neufeld KM, Kang N, Bienenstock J, Foster JA (2011) Reduced anxiety‐like behavior and central neurochemical change in germ‐free mice. Neurogastroenterol Motil 23(3):255-e119.
Noback CRSNL, Demarest RJ (1995) The human nervous system. Philadelphia: Williams and Wilkins
Noble JM, Scarmeas N, Celenti RS et al (2014) Serum IgG antibody levels to periodontal microbiota are associated with incident Alzheimer disease. PLoS One 9:e114959
Obrenovich M, Siddiqui B, McCloskey B, Reddy VP (2020) The Microbiota–Gut–Brain Axis Heart Shunt Part I: The French Paradox, Heart Disease and the Microbiota. Microorganisms 8(4):490
Osborne G, Wu F, Yang L, Kelly D, Hu J, Li H, Jasmine F, Kibriya MG, Parvez F, Shaheen I, Sarwar G (2020) The association between gut microbiome and anthropometric measurements in Bangladesh. Gut microbes 11(1):63–76
Ozaki Y, Yoshino Y, Yamazaki K, Sao T, Mori Y, Ochi S, Yoshida T, Mori T, Iga JI, Ueno SI (2017) DNA methylation changes at TREM2 intron 1 and TREM2 mRNA expression in patients with Alzheimer’s disease. J Psychiatr Res 92:74–80
Pacheco AR, Barile D, Underwood MA, Mills DA (2015) The impact of the milk glycobiome on the neonate gut microbiota. Annu Rev Anim Biosci 16:3(1):419–45
Pan W, Zhu S, Yuan M, Cui H, Wang L, Luo X et al (2010) MicroRNA-21 and microRNA-148a contribute to DNA hypomethylation in lupus CD4+ T cells by directly and indirectly targeting DNA methyltransferase 1. J Immunol 184(12):6773–6781
Paoletti P, Bellone C, Zhou Q (2013) NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci 14(6):383–400
Parashar A, Mehta V, Udayabanu M (2017) Rutin alleviates chronic unpredictable stress-induced behavioral alterations and hippocampal damage in mice. Neurosci Lett 656:65–71
Parashar A, Udayabanu M (2017) Gut microbiota: Implications in Parkinson’s disease. Parkinsonism Relat Disord 38:1–7
Pasciuto E, Burton OT, Roca CP, Lagou V, Rajan WD, Theys T, Mancuso R, Tito RY, Kouser L, Callaerts-Vegh Z, Alerie G (2020) Microglia require CD4 T cells to complete the fetal-to-adult transition. Cell 182(3):625–640
Pastor WA, Aravind L, Rao A (2013) TETonic shift: biological roles of TET proteins in DNA demethylation and transcription. Nat Rev Mol Cell Biol 14(6):341–356
Pazin MJ, Kadonaga JT (1997) What’s up and down with histone deacetylation and transcription? Cell 89(3):325–328
Perrig WJ, Perrig P, Stähelin HB (1997) The relation between antioxidants and memory performance in the old and very old. J Am Geriatr Soc 45:718–724
Picklo MJ, Montine TJ, Amarnath V, Neely MD (2002) Carbonyl toxicology and Alzheimer’s disease. Toxicol Appl Pharmacol 184:187–197
Pistollato F, Sumalla Cano S, Elio I, Masias Vergara M, Giampieri F, Battino M (2016) Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr Rev 74(10):624–634
Plagg B, Ehrlich D, Kniewallner KM, Marksteiner J, Humpel C (2015) Increased acetylation of histone H4 at lysine 12 (H4K12) in monocytes of transgenic Alzheimer’s mice and in human patients. Curr Alzheimer Res 12(8):752–760
Poole S, Singhrao SK, Kesavalu L, Curtis MA, Crean S (2013) Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer’s disease brain tissue. J Alzheimers Dis 36:665–677
Pop M (2012) We are what we eat: how the diet of infants affects their gut microbiome. Genome Biol 13:152
Ramassamy C, Krzywkowski P, Averill D, Lussier-Cacan S, Theroux L, Christen Y et al (2001) Impact of ApoE deficiency on oxidative insults and antioxidant levels in the brain. Brain Res Mol Brain Res 86:76–83
Reddy DS, Wu X, Golub VM, Dashwood WM, Dashwood RH (2018) Measuring histone deacetylase inhibition in the brain. Curr Protoc Pharmacol 81(1):e41
Reid MA, Dai Z, Locasale JW (2017) The impact of cellular metabolism on chromatin dynamics and epigenetics. Nat Cell Biol 19(11):1298–1306
Rhee SH, Pothoulakis C, Mayer EA (2009) Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol 6:306–314
Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GA, Gasbarrini A, Mele MC (2019) What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 7(1):14
Rogers J, Cooper NR, Webster S, Schultz J, McGeer PL, Styren SD et al (1992) Complement activation by b-amyloid in Alzheimer disease. Proc Natl Acad Sci USA 89:10016–10020
Rosenbaum M, Knight R, Leibel RL (2015) The gut microbiota in human energy homeostasis and obesity. Trends Endocrinol Metab 26(9):493–501
Rösler M, Retz W, Thome J, Riederer P (1998) Free radicals in Alzheimer’s dementia: currently available therapeutic strategies. J Neural Transm 54:211–219
Roth SY, Denu JM, Allis CD (2001) Histone acetyltransferases. Annu Rev Biochem 70(1):81–120
Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D et al (2018) Environment dominates over host genetics in shaping human gut microbiota. Nature 555(7695):210–215
Roubaud-Baudron C, Krolak-Salmon P, Quadrio I, Mégraud F, Salles N (2012) Impact of chronic Helicobacter pylori infection on Alzheimer’s disease: preliminary results. Neurobiol Aging 33:1009
Sandler RH, Finegold SM, Bolte ER, Buchanan CP, Maxwell AP, Väisänen ML et al (2000) Short-term benefit from oral vancomycin treatment of regressive-onset autism. J Child Neurol 15:429–435
Santoro A, Ostan R, Candela M, Biagi E, Brigidi P, Capri M et al (2018) Gut microbiota changes in the extreme decades of human life: a focus on centenarians. Cell Mol Life Sci 75:129–148
Santos F, Wegkamp A, de Vos WM, Smid EJ, Hugenholtz J (2008) High-Level folate production in fermented foods by the B12 producer Lactobacillus reuteri JCM1112. Appl Environ Microbiol 74:3291–3294
Savignac HM, Tramullas M, Kiely B, Dinan TG, Cryan JF (2015) Bifidobacteria modulate cognitive processes in an anxious mouse strain. Behav Brain Res 287:59–72
Sayre LM, Moreira PI, Smith MA, Perry G (2005) Metal ions and oxidative protein modification in neurological disease. Ann Ist Super Sanita 41:143–164
Sayre LM, Perry G, Smith MA (2008) Oxidative stress and neurotoxicity. Chem Res Toxicol 21:172–188
Schippling S, Kontush A, Arlt S, Buhmann C, Stürenburg HJ, Mann U et al (2000) Increased lipoprotein oxidation in Alzheimer’s disease. Free Radic Biol Med 28:351–360
Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D (1999) Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400(6740):173–177
Schulz JB, Lindenau J, Seyfried J, Dichgans J (2000) Glutathione, oxidative stress and neurodegeneration. Eur J Biochem 267(16):4904–4911
Schwartz S, Friedberg I, Ivanov IV, Davidson LA, Goldsby JS, Dahl DB et al (2012) A metagenomic study of diet-dependent interaction between gut microbiota and host in infants reveals differences in immune response. Genome Biol 13:r32
Selkrig J, Wong P, Zhang X, Pettersson S (2014) Metabolic tinkering by the gut microbiome: implications for brain development and function. Gut Microbes 5(3):369–380
Shahbazian MD, Grunstein M (2007) Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem 76:75–100
Sharma VK, Mehta V, Singh GT (2020) Alzheimer’s Disorder: Epigenetic Connection and Associated Risk Factors. Curr Neuropharmacol (In press)
Sharon G, Sampson TR, Geschwind DH, Mazmanian SK (2016) The central nervous system and the gut microbiome. Cell 167:915–932
Shenderov BA (2012) Gut indigenous microbiota and epigenetics. Microb Ecol Health Dis 23(1):17195
Shi LH, Balakrishnan K, Thiagarajah K, Ismail NI, Yin OS (2016) Beneficial properties of probiotics. Trop Life Sci Res 27(2):73
Silva YP, Bernardi A, Frozza RL (2020) The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol 11:25
Simard AR, Soulet D, Gowing G, Julien JP, Rivest S (2006) Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron 49(4):489–502
Skaper DS, Facci L, Zusso M, Giusti P (2017) Synaptic plasticity, dementia and Alzheimer disease. CNS Neurol Disord Drug Targets 16(3):220–233
Smythies LE, Shen R, Bimczok D, Novak L, Clements RH, Eckhoff DE, Bouchard P, George MD, Hu WK, Dandekar S, Smith PD (2010) Inflammation anergy in human intestinal macrophages is due to Smad-induced IκBα expression and NF-κB inactivation. J Biol Chem 285(25):19593–19604
Sochocka M, Donskow-Łysoniewska K, Diniz BS, Kurpas D, Brzozowska E, Leszek J (2019) The gut microbiome alterations and inflammation-driven pathogenesis of Alzheimer’s disease—a critical review. Mol Neurobiol 56(3):1841–1851
Sparks Stein P, Steffen MJ, Smith C et al (2012) Serum antibodies to periodontal pathogens are a risk factor for Alzheimer’s disease. Alzheimers Dement
Stilling RM, Dinan TG, Cryan JF (2014) Microbial genes, brain & behaviour–epigenetic regulation of the gut–brain axis. Genes Brain Behav 13(1):69–86
Sullivan R, Wilson DA, Feldon J, Yee BK, Meyer U, Richter-Levin G et al (2006) The International Society for Developmental Psychobiology annual meeting symposium: Impact of early life experiences on brain and behavioral development. Dev Psychobiol 48:583–602
Sung YM, Lee T, Yoon H, DiBattista AM, Song JM, Sohn Y et al (2013) Mercaptoacetamide-based class II HDAC inhibitor lowers Aβ levels and improves learning and memory in a mouse model of Alzheimer’s disease. Exp Neurol 239:192–201
Syed AK, Boles BR (2014) Fold modulating function: Bacterial toxins to functional amyloids. Front Microbiol 5:401
Tönnies E, Trushina E (2017) Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J Alzheimers Dis 57(4):1105–1121
Tse JK (2017) Gut microbiota, nitric oxide, and microglia as prerequisites for neurodegenerative disorders. ACS Chem Neurosci 8(7):1438–1447
Van Noort JM, Bsibsi M (2009) Toll-like receptors in the CNS: implications for neurodegeneration and repair. Prog Brain Res 175:139–148
Virarkar M, Alappat L, Bradford PG, Awad AB (2013) L-arginine and nitric oxide in CNS function and neurodegenerative diseases. Crit Rev Food Sci Nutr 53(11):1157–1167
Vogt NM, Kerby RL, Dill-McFarland KA et al (2017) Gut microbiome alterations in Alzheimer’s disease. Shci Rep 7:13537. https://doi.org/10.1038/s41598-017-13601-y
Wang X, Zhu M, Hjorth E, Cortés-Toro V, Eyjolfsdottir H, Graff C et al (2015a) Resolution of inflammation is altered in Alzheimer’s disease. Alzheimers Dement 11(1):40–50
Wang XL, Zeng J, Yang Y, Xiong Y, Zhang ZH, Qiu M (2015b) Helicobacter pylori filtrate induces Alzheimer-like tau hyperphosphorylation by activating glycogen synthase kinase-3β. J Alzheimers Dis 43:153–165
Watson CT, Roussos P, Garg P, Ho DJ, Azam N, Katsel P et al (2016) Genome-wide DNA methylation profiling in the superior temporal gyrus reveals epigenetic signatures associated with Alzheimer’s disease. Genome Med 8(1):5
Watt R, Parkin K, Martino D (2020) The Potential Effects of Short-Chain Fatty Acids on the Epigenetic Regulation of Innate Immune Memory. Challenges 11(2):25
Weggen S, Beher D (2012) Molecular consequences of amyloid precursor protein and presenilin mutations causing autosomal-dominant Alzheimer’s disease. Alzheimers Res Ther 4(2):9
Westfall S, Lomis N, Kahouli I, Dia SY, Singh SP, Prakash S (2017) Microbiome, probiotics and neurodegenerative diseases: deciphering the gut brain axis. Cell Mol Life Sci 74(20):3769–3787
Williams AM, Probert CS, Stepankova R, Tlaskalova-Hogenova H, Phillips A, Bland PW (2006) Effects of microflora on the neonatal development of gut mucosal T cells and myeloid cells in the mouse. Immunology 119(4):470–478
Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D (2010) Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 32(6):815–827
Wu HJ, Wu E (2012) The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 3(1):4–14
Xu R, Wang Q (2016) towards understanding brain-gut-microbiome connections in Alzheimer’s disease. BMC Syst Biol 10:63
Yang D, Zhao D, Shah SZ, Wu W, Lai M, Zhang X et al (2019) The Role of the gut microbiota in the pathogenesis of Parkinson's disease. Front Neurol 10
Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M et al (2012) Human gut microbiome viewed across age and geography. Nature 486(7402):222–227
Ye J, Wu W, Li Y, Li L (2017) Influences of the gut microbiota on DNA methylation and histone modification. Dig Dis Sci 62(5):1155–1164
Youssef NH, Couger MB, McCully AL, Criado AE, Elshahed MS (2015) Assessing the global phylum level diversity within the bacterial domain: a review. J Adv Res 6(3):269–282
Yu CC, Jiang T, Yang AF, Du YJ, Wu M, Kong LH (2019) Epigenetic modulation on tau phosphorylation in Alzheimer’s disease. Neural plasticity 2019
Zawia NH, Lahiri DK, Cardozo-Pelaez F (2009) Epigenetics, oxidative stress, and Alzheimer disease. Free Radic Biol Med 46(9):1241–1249
Zhan X, Stamova B, Jin L-W, DeCarli C, Phinney B, Sharp FR (2016) Gram-negative bacterial molecules associate with Alzheimer disease pathology. Neurology 87(22):2324–2332
Zhan X, Stamova B, Sharp FR (2018) Lipopolysaccharide associates with amyloid plaques, neurons and oligodendrocytes in Alzheimer’s disease brain: a review. Front Aging Neurosci 10:42
Zhang R, Miller RG, Gascon R, Champion S, Katz J, Lancero M, Narvaez A, Honrada R, Ruvalcaba D, McGrath MS (2009) Circulating endotoxin and systemic immune activation in sporadic amyotrophic lateral sclerosis (sALS). J Neuroimmunol 206(1-2):121-4.
Zhao Y, Jaber V, Lukiw WJ (2017) Secretory products of the human GI tract microbiome and their potential impact on Alzheimer’s disease (AD): detection of lipopolysaccharide (LPS) in AD hippocampus. Front Cell Infect Microbiol 7:318
Zhao Y, Lukiw WJ (2018) Bacteroidetes neurotoxins and inflammatory neurodegeneration. Mol Neurobiol 55(12):9100–9107
Zhu S, Jiang Y, Xu K, Cui M, Ye W, Zhao G et al (2020) The progress of gut microbiome research related to brain disorders. J Neuroinflammation 17(1):25
Zhuang ZQ, Shen LL, Li WW, Fu X, Zeng F, Gui L, Lü Y, Cai M, Zhu C, Tan YL, Zheng P (2018) Gut microbiota is altered in patients with Alzheimer’s disease. J Alzheimers Dis 63(4):1337–1346
Ziech D, Franco R, Pappa A, Panayiotidis MI (2011) Reactive Oxygen Species (ROS)––Induced genetic and epigenetic alterations in human carcinogenesis. Mutat Res 711(1–2):167–173
Zmora N, Suez J, Elinav E (2019) You are what you eat: diet, health and the gut microbiota. Nat Reviews Gastroenterol Hepatol 16(1):35–56
Zusso M, Barbierato M, Facci L, Skaper SD, Giusti P (2018) Neuroepigenetics and Alzheimer’s disease: an update. J Alzheimers Dis 64(3):671–688
Acknowledgments
The authors would like to acknowledge the Govt. College of Pharmacy, Shoolini University of Biotechnology and Management Sciences for supporting this work.
Author information
Authors and Affiliations
Contributions
PN and VM designed and planned the manuscript. PN was involved in collecting literature and designing the manuscript’s first draft. TB and AP provided technical input and were involved in extensive manuscript editing. VM edited the manuscript, provided technical inputs, and finalized the draft for publication.
Corresponding author
Ethics declarations
Consent for Publication
All the authors agree to publish this manuscript without any conflicts
Competing Interest
The authors have no competing interests or conflicts for the publication of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nagu, P., Parashar, A., Behl, T. et al. Gut Microbiota Composition and Epigenetic Molecular Changes Connected to the Pathogenesis of Alzheimer’s Disease. J Mol Neurosci 71, 1436–1455 (2021). https://doi.org/10.1007/s12031-021-01829-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-021-01829-3