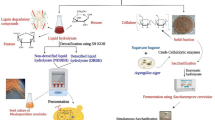
Abstract
Investigation of an alternative source of the nonrenewable energy is attracting the world attention nowadays. The fruits and leaves biomass of Calotropis procera were exposed to sequential pretreatment processes. First, a successive pretreatment with an organic solvent (hexane/methanol) yielded biocrude and plant spent residues fractions. The plant spent residues fractions were exposed to successive acid/alkali (1% H2SO4/2% NaOH) pretreatment to yield spent residues of fruits (SRF) and leaves (SRL). The amount of cellulose content of the sequential pretreated SRF and SRL were 87.3 and 83.4%, respectively. The hydrolysis process of 10 g of the sequential pretreated SRF and SRL by Sternzym cellulase produced 80.2 and 50.4 g/L of total reduced sugars, respectively. The reduced sugars were then fermented by Saccharomyces cerevisiae (MN901244) for bioethanol production. The HPLC analysis showed 38.9 and 23.8 g/L of bioethanol yields from sequential pretreated SRF and SRL, respectively. The GC/MS analysis of methanol and hexane biocrude fractions revealed the presence of 20 and 12% of fatty acids, respectively, which would be promising amounts for biodiesel production after esterification. So, the present study suggested a low-cost nonedible plant biomass (Calotropis procera) as a biorefinery for production of renewable bioethanol.
Graphical abstract






Similar content being viewed by others
References
Phwan CK, Ong HC, Chen W-H, Ling TC, Ng EP, Show PL (2018) Overview: comparison of pretreatment technologies and fermentation processes of bioethanol from microalgae. Energy Convers Manag 173:81–94
Bu J, Yan X, Wang Y-T, Zhu S-M, Zhu M-J (2019) Co-production of high-gravity bioethanol and succinic acid from potassium peroxymonosulfate and deacetylation sequentially pretreated sugarcane bagasse by simultaneous saccharification and co-fermentation. Energy Convers Manag 186:131–139
Balat M (2011) Production of bioethanol from lignocellulosic materials via the biochemical pathway: a review. Energy Convers Manag 52:858–875
Xu S, Elsayed M, Ismail GA, Li C, Wang S, Abomohra AE-F (2019) Evaluation of bioethanol and biodiesel production from Scenedesmus obliquus grown in biodiesel waste glycerol: a sequential integrated route for enhanced energy recovery. Energy Convers Manag 197:111907
de Carvalho DM, Sevastyanova O, de Queiroz JH, Colodette JL (2016) Cold alkaline extraction as a pretreatment for bioethanol production from eucalyptus, sugarcane bagasse and sugarcane straw. Energy Convers Manag 124:315–324
Fatih D, Balat M, Balat H (2011) Biowastes-to-biofuels. Energy Convers Manag 52:1815–1828
Demirbas A (2009) Political, economic and environmental impacts of biofuels: a review. Appl Energy 86:S108–SS17
El-Dalatony MM, Salama E-S, Kurade MB, Hassan SH, Oh S-E, Kim S et al (2017) Utilization of microalgal biofractions for bioethanol, higher alcohols, and biodiesel production: a review. Energies 10:2110
Demirbaş A (2005) Bioethanol from cellulosic materials: a renewable motor fuel from biomass. Energy Sources 27:327–337
Kumar G, Sivagurunathan P, Kim S-H, Bakonyi P, Lin C-Y (2015) Modeling and optimization of biohydrogen production from de-oiled Jatropha using the response surface method. Arab J Sci Eng 40:15–22
Chakrabarti MH, Ali M, Usmani JN, Baroutian S, Saleem M (2013) Technical evaluation of pongame and Jatropha B20 fuels in Pakistan. Arab J Sci Eng 38:759–766
Ashokkumar V, Salim MR, Salam Z, Sivakumar P, Chong CT, Elumalai S, Suresh V, Ani FN (2017) Production of liquid biofuels (biodiesel and bioethanol) from brown marine macroalgae Padina tetrastromatica. Energy Convers Manag 135:351–361
Ofori-Boateng C, Lee KT (2014) Same-vessel enzymatic saccharification and fermentation of organosolv/H2O2 pretreated oil palm (Elaeis guineensis Jacq.) fronds for bioethanol production: optimization of process parameters. Energy Convers Manag 78:421–430
Kuttiraja M, Sindhu R, Varghese PE, Sandhya SV, Binod P, Vani S, Pandey A, Sukumaran RK (2013) Bioethanol production from bamboo (Dendrocalamus sp.) process waste. Biomass Bioenergy 59:142–150
Mikulski D, Kłosowski G (2020) Microwave-assisted dilute acid pretreatment in bioethanol production from wheat and rye stillages. Biomass Bioenergy 136:105528
Azhar SHM, Abdulla R, Jambo SA, Marbawi H, Gansau JA, Faik AAM et al (2017) Yeasts in sustainable bioethanol production: a review. Biochem Biophys Reports 10:52–61
Hassan SH, Abd el Nasser AZ, Kassim RM (2019) Electricity generation from sugarcane molasses using microbial fuel cell technologies. Energy 178:538–543
Elsayed M, Abomohra AE-F, Ai P, Jin K, Fan Q, Zhang Y (2019) Acetogenesis and methanogenesis liquid digestates for pretreatment of rice straw: a holistic approach for efficient biomethane production and nutrient recycling. Energy Convers Manag 195:447–456
Alvira P, Tomás-Pejó E, Ballesteros M, Negro M (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 101:4851–4861
Datta R (1981) Acidogenic fermentation of lignocellulose–acid yield and conversion of components. Biotechnol Bioeng 23:2167–2170
Pesce GR, Fernandes MC, Mauromicale G (2020) Globe artichoke crop residues and their potential for bioethanol production by dilute acid hydrolysis. Biomass Bioenergy 134:105471
Tawfik A, El-Bery H, Elsamadony M, Kumari S, Bux F (2019) Upgrading continuous H2 gas recovery from rice straw hydrolysate via fermentation process amended with magnetite nanoparticles. Int J Energy Res 43:3516–3527
Yamada R, Wakita K, Ogino H (2017) Global metabolic engineering of glycolytic pathway via multicopy integration in Saccharomyces cerevisiae. ACS Synth Biol 6:659–666
Kalita D, Saikia C (2004) Chemical constituents and energy content of some latex bearing plants. Bioresour Technol 92:219–227
Padmaja K, Atheya N, Bhatnagar A, Singh K (2009) Conversion of Calotropis procera biocrude to liquid fuels using thermal and catalytic cracking. Fuel 88:780–785
Mutwakil MZ, Hajrah NH, Atef A, Edris S, Sabir MJ, Al-Ghamdi AK et al (2017) Transcriptomic and metabolic responses of Calotropis procera to salt and drought stress. BMC Plant Biol 17:231
Garg J, Kumar A (2013) Some potential biofuel plants for production of biodiesel in semi-arid and arid conditions: a review. African J Plant Sci 7:124–127
Maroyi A (2012) Calotropis procera (Aiton) WT Aiton. In: Schmelzer GH, Gurib-Fakim A (eds) Prota 11(2). Medicinal plants/Plantes médicinales 2. PROTA, Wageningen, Pays Bas. Consulté le
Sousa LV, Santos APB, Souza LD, Santos AGD, Beatriz A (2018) Evaluation of the properties of Calotropis procera oil aiming the production of biodiesel. Orbital: Electron J Chem 10:147–152
Behera B, Arora M, Sharma D (1996) Scanning electron microscopic (SEM) studies on structural architecture of lignocellulosic materials of Calotropis procera during its processing for saccharification. Bioresour Technol 58:241–245
Rezende CA, de Lima MA, Maziero P, de Azevedo ER, Garcia W, Polikarpov I (2011) Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility. Biotechnol Biofuels 4:54
Ververis C, Georghiou K, Danielidis D, Hatzinikolaou D, Santas P, Santas R et al (2007) Cellulose, hemicelluloses, lignin and ash content of some organic materials and their suitability for use as paper pulp supplements. Bioresour Technol 98:296–301
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Yousef N, Mawad A, Abeed A (2019) Enhancement the cellulase activity induced by endophytic bacteria using calcium nanoparticles. Curr Microbiol 76:346–354
Farghaly A, Elsamadony M, Ookawara S, Tawfik A (2017) Bioethanol production from paperboard mill sludge using acid-catalyzed bio-derived choline acetate ionic liquid pretreatment followed by fermentation process. Energy Convers Manag 145:255–264
Hashem M, Hesham AE-L, Alrumman SA, Alamri SA, Moustafa MF (2014) Indigenous yeasts of the rotten date fruits and their potentiality in bioethanol and single-cell protein production. Int J Agric Biol 16:752–758
Nuwamanya E, Chiwona-Karltun L, Kawuki RS, Baguma Y (2012) Bio-ethanol production from non-food parts of cassava (Manihot esculenta Crantz). Ambio 41:262–270
Gonçalves FA, Ruiz HA, da Costa NC, dos Santos ES, Teixeira JA, de Macedo GR (2014) Comparison of delignified coconuts waste and cactus for fuel-ethanol production by the simultaneous and semi-simultaneous saccharification and fermentation strategies. Fuel 131:66–76
Araque E, Parra C, Freer J, Contreras D, Rodríguez J, Mendonça R, Baeza J (2008) Evaluation of organosolv pretreatment for the conversion of Pinus radiata D. Don to ethanol. Enzym Microb Technol 43:214–219
Saha BC (2003) Hemicellulose bioconversion. J Ind Microbiol Biotechnol 30:279–291
Meryemoğlu B, Hasanoglu A, Kurtulus M, Irmak S (2018) Investigation of organic solvents effects on Kenaf (Hibiscus cannabinus L.) biomass conversion in subcritical water. Journal of the Turkish Chemical Society. Sect A: Chem 5:1423–1430
Talebnia F, Karakashev D, Angelidaki IJB (2010) Production of bioethanol from wheat straw: an overview on pretreatment, hydrolysis and fermentation. Bioresour Technol 101:4744–4753
Moeini S, Dadashian F, Vahabzadeh F (2016) Recycling of Calotropis gigantea waste fiber by enzymatic hydrolysis to produce bioethanol. Indian J Sci Technol 9:1–6
Bhagia S, Li H, Gao X, Kumar R, Wyman CE (2016) Flowthrough pretreatment with very dilute acid provides insights into high lignin contribution to biomass recalcitrance. Biotechnol Biofuels 9:245
Maurya DP, Singla A, Negi S (2015) An overview of key pretreatment processes for biological conversion of lignocellulosic biomass to bioethanol. 3. Biotech 5:597–609
Chandra RP, Bura R, Mabee W, Berlin DA, Pan X, Saddler J (2007) Substrate pretreatment: the key to effective enzymatic hydrolysis of lignocellulosics? Biofuels: Springer 108:67–93
Amoah J, Ogura K, Schmetz Q, Kondo A, Ogino C (2019) Co-fermentation of xylose and glucose from ionic liquid pretreated sugar cane bagasse for bioethanol production using engineered xylose assimilating yeast. Biomass Bioenergy 128:105283
Abdullah B, Muhammad SAF, Shokravi Z, Ismail S, Kassim KA, Mahmood AN et al (2019) Fourth generation biofuel: A review on risks and mitigation strategies. Renew Sust Energ Rev 107:37–50
Mussatto SI, Machado EM, Carneiro LM, Teixeira JA (2012) Sugars metabolism and ethanol production by different yeast strains from coffee industry wastes hydrolysates. Appl Energy 92:763–768
Kumari R, Pramanik K (2013) Bioethanol production from Ipomoea carnea biomass using a potential hybrid yeast strain. Appl Biochem Biotechnol 171:771–785
Shafiei M, Zilouei H, Zamani A, Taherzadeh MJ, Karimi K (2013) Enhancement of ethanol production from spruce wood chips by ionic liquid pretreatment. Appl Energy 102:163–169
Saha BC, Yoshida T, Cotta MA, Sonomoto K (2013) Hydrothermal pretreatment and enzymatic saccharification of corn stover for efficient ethanol production. Ind Crop Prod 44:367–372
El-Bery H, Tawfik A, Kumari S, Bux F (2013) Effect of thermal pre-treatment on inoculum sludge to enhance bio-hydrogen production from alkali hydrolysed rice straw in a mesophilic anaerobic baffled reactor. Environ Technol 34:1965–1972
Nguyen CM, Nguyen TN, Choi GJ, Choi YH, Jang KS, Park Y-J, Kim JC (2014) Acid hydrolysis of Curcuma longa residue for ethanol and lactic acid fermentation. Bioresour Technol 151:227–235
Harun R, Jason WSY, Cherrington T, Danquah MK (2011) Exploring alkaline pre-treatment of microalgal biomass for bioethanol production. Appl Energy 88(10):3464–3467
Moon M, Kim CW, Farooq W, Suh WI, Shrivastav A, Park MS, Mishra SK, Yang JW (2014) Utilization of lipid extracted algal biomass and sugar factory wastewater for algal growth and lipid enhancement of Ettlia sp. Bioresour Technol 163:180–185
Wilkes M, Takei I, Caldwell R, Trethowan R (2013) The effect of genotype and environment on biodiesel quality prepared from Indian mustard (Brassica juncea) grown in Australia. Ind Crop Prod 48:124–132
Acknowledgements
The authors would like to thank Dr. Ibrahim M. A. Nafady the director of Wadi Al-Asiuty protected area for helping in collecting the Calotropis procera plant. Also, this work was supported by Science, Technology & Innovation Funding Authority (STIFA) in Egypt as a part of research projects (ID: 41623,43281).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mahmoud, A.H., El-Bery, H.M., Ali, M.M. et al. Latex-bearing plant (Calotropis procera) as a biorefinery for bioethanol production. Biomass Conv. Bioref. 13, 4785–4795 (2023). https://doi.org/10.1007/s13399-021-01479-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01479-w