Abstract
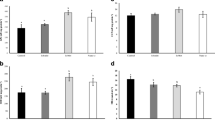
The purpose of this study was to investigate the effect of commercial Lactobacillus probiotic and iron nanoparticles on some blood biochemical parameters of common carp (Cyprinus carpio). Common carp (mean weight 50 g) was fed in six treatments with 0.25 and 0.50 mg/g iron oxide nanoparticles and 108 CFU/g commercial Lactobacillus probiotic either together or separately for 56 days. After the rearing period, blood biochemical parameters including total plasma protein, albumin, globulin, creatinine, triglyceride and cholesterol and ALP, ALT, AST and LDH enzymes were measured. Total protein and globulin levels showed a significant increase in probiotic alone and probiotic plus 0.50 mg iron nanoparticles treatments (p ≤ 0.05). Creatinine, albumin, liver enzymes, serum lipids did not show any significant differences between treatments (p ≥ 0.05), but the values of these parameters showed no negative effects in different treatments. Based on the findings of this study, it can be concluded that using iron nanoparticles and Lactobacillus probiotic can improve some of the biochemical factors in carp.





Similar content being viewed by others
Change history
17 December 2021
An Erratum to this paper has been published: https://doi.org/10.1134/S1062359021060078
REFERENCES
Afkhami-Ardakani, M., Shirband, A., Golzade, J., Asadi-Samani, M., Latifi, E., and Kheylapour, M., The effect of iron oxide nanoparticles on liver enzymes (ALT, AST and ALP), thyroid hormones (T3 and T4) and TSH in rats, J. Shahr. Univ. Med. Sci., 2013, vol.14, pp. 82–88.
Akbary, P. and Jahanbakhshi, A., Nano and macro iron oxide (Fe2O3) as feed additives: effects on growth, biochemical, activity of hepatic enzymes, liver histopathology and appetite-related gene transcript in goldfish (Carassius auratus), Aquaculture, 2019, vol. 510, pp. 191–197.
Andrews, S.R., Sahu, N.P., Pal, A.K., Mukherjee, S.C., and Kumar, S., Yeast extract, brewer’s yeast and spirulina in diets for Labeo rohita fingerlings affect haematoimmunological responses and survival following Aeromonas hydrophila challenge, J. Res. Vet. Sci., 2011, vol. 91, pp. 103–109.
Aitken, R.J., Chaudhry, M.Q., Boxall, A.B.A., and Hull, M., Manufacture and use of nanomaterials: current status in the UK and global trends, J. Occup. Med. Environ. Health, 2006, vol. 56, pp. 300–330.
Bandyopadhyay, P. and Mohapatra, P.K.D., Effect of a probiotic bacterium Bacillus circulans PB7 in the formulated diets: on growth, nutritional quality and immunity of Catla catla (Ham.), J. Fish Physiol. Biochem., 2009, vol. 35, pp. 467–478.
Beard, J., Felt, B., Schallert, T., Burhans, M., Connor, J.R., and Georgieff, M.K., Moderate iron deficiency in infancy, J. Behav. Bras. Ref., 2006, vol. 170, pp. 223–224.
Boshra, H., Li, J., and Sunyer, J.O., Recent advances on the complement system of teleost fish, J. Fish. Shell. Immunol., 2006, vol. 20, pp. 239–262.
Chen, C.Y., Gregory,A. and Wooster, P.R., Bowser comparative blood chemistry and histopathology; tilapia infected with Vibrio vulnificus or Streptococcus iniae or exposed to carbon tetrachloride, gentamicin, or copper sulfate, J. Aquacult., 2004, vol. 239, pp. 421–443.
Dugenci, S. K., Arda, N. and Cand, A., Some medicinal plants as immuno stimulants for fish, J. Ethnoph., 2003, vol.88, pp. 99–106.
Fuller, R., History and Development of Probiotics, Fuller, R., Ed., Dordrecht: Springer, 1992.
Gatesoupe, F.J. and Ringo, E., Lactic acid bacteria in fish: a review, J. Aquacult., 1998, vol. 160, pp. 177–203.
Gatlin, D.M., Nutrition and Fish Health, Halver, J.E. and Hardy, R.W., Eds., San Diego: Academic, 2002.
Ghasempour Dehaghani, P., Javaheri Baboli, M., Taghavi Moghadam, A., Ziaei-Nejad, S., and Pourfarhadi M., Effect of synbiotic dietary supplementation on survival, growth performance, and digestive enzyme activities of common carp (Cyprinus carpio) fingerlings, Czech J. Anim. Sci., 2015, vol. 60, pp. 224–232.
Gupta, A.K., Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications, J. Biomat., 2005, vol. 26, pp. 3995–4021.
Hasanpour Fattahi, A., Jafaryan, H., and Khosravi, A., The combined effects of the yeast Saccharomyces cerevisiae and Aspergillus niger on the haematological and biochemical parameters of cultured juvenile beluga (Huso huso), J. Veter. Res., 2015, vol. 70, pp. 463–473.
Hernandez, L.H.H., Teshima, S.I., Koshio, S., Ishikawa, M., and Tanaka, Y.M.S., Effects of vitamin A on growth, serum anti-bacterial activity and transaminase activities in the juvenile Japanese flounder, Paralichthys olivaceus, J. Aquacult., 2007, vol. 262, pp. 444–450.
Jha, A.K., Pal, A.K., Sahu, N.P., Kumar, S., and Mukherjee, S.C., Haematoimmunological responses to dietary yeast RNA, n-3 fatty acid and b carotene in Catla catla juveniles, J. Fish. Shell. Immunol., 2007, vol. 23, pp. 917–927.
Jiang, J., Oberdörster, G., and Biswas, P., Characterization of size, surface charge, and agglomeration state of nano particle dispersions for toxicological studies, Nano. Res., 2008, vol. 11, pp. 77–89.
Kazuń, B., Małaczewska, J., Kazuń, K., Żylińska-Urban, J., and Siwicki, A.K., Immune-enhancing activity of potential probiotic strains of Lactobacillus plantarum in the common carp (Cyprinus carpio) fingerling, J. Vet. Res., 2018, vol. 62, pp. 485–492.
Khoshghalb, M., Azari Takami, G., Khara, H., and Khazemi, R., Effects of different levels of bactocell supplemented with diet on some immunological parameters in rainbow trout (Oncorhynchus mykiss), J. Physiol. Anim. Dev., 2013, vol. 6, pp. 53–66.
Mohammadi, N. and Tukmechi, A., The effects of iron nanoparticles in combination with Lactobacillus casei on growth parameters and probiotic counts in rainbow trout (Oncorhynchus mykiss) intestine, J. Vet. Res., 2015, vol. 70, pp. 47–53.
Nayak, S.K., Probiotics and immunity: a fish perspective, Fish Shellfish Immunol., 2010, vol. 29, pp. 2–14.
Nya, E.J. and Austin, B., Use of garlic, Allium sativum, to control Aeromonas hydrophila infection in rainbow trout, Oncorhynchus mykiss (Walbaum), J. Fish Dis., 2009, vol. 32, pp. 963–970.
Prochorov, A.M., Pavlov, G.V., Godwin, A.C., and Okpattah, K.A.V., Nanotechnology in agriculture and food production, J. Appl. Environ. Biol. Sci., 2011, vol. 1, pp. 414–419.
Racicot, J.G., Gaudet, M., and Leray, C., Blood and liver enzymes in rainbow trout (Salmo gairdneri) with emphasis on their diagnostic use: study of CCl4 toxicity and a case of Aeromonas infection, J. Fish Biol., 1975, vol. 7, pp. 825–835.
Rehulka, J., Blood indices of the rainbow trout (Oncohynchus mykiss) in Aeromonas induced ulcerous dermatitis, J. Ac. Vet., 1998, vol. 67, pp. 317–322.
Reid, R.T., Live, D.H., Faulkner, D.J., and Butler, A., A siderophore from a marine bacterium with an exceptional ferric ion affinity constant, Nature, 1993, vol. 366, pp. 455–458.
Rezaei, F., Jamili, S., Ehteshami, F., Mashinchian Moradi, A., and Shahidian Namghi, M., Red blood cells of fish Cyprinus carpio, J. Anim. Environ., 2014, vol. 6, pp. 197–202.
Sancho, E., Ferrando, M.D., and Andrev, E., Sublethal effects of an organophosphate insectidide on the European eel, Anguilla Anguilla, J. Ecotoxicol. Environ. Saf., 1997, vol. 36, pp. 57–65.
Shahbazi, P. and Maleknia, N., General Biochemistry for Students of Medical Sciences, Tehran: Tehran Univ. Press, 2004.
Soltani, M., Abdy, E., Alishahi, M., Taheri Mirghaed, A., and Hosseini-Shekarabi, P., Growth performance, immune-physiological variables and disease resistance of common carp (Cyprinus carpio) orally subjected to different concentrations of Lactobacillus plantarum, Aquacult. Int., 2017, vol. 25, pp. 1913–1933.
Tavana, M., Kalbassi, M.R., Abedian Kenari, A., and Johari, S.A., Assessment of assimilation and elimination of silver and TiO2 nanoparticles in Artemia franciscana in different salinities, J. Oceanol., 2014, vol. 5, pp. 91–103.
Thangapandiyan, S., Alif Alisha, A.S., and Anidha, K., Growth performance, hematological and biochemical effects of iron oxide nanoparticles in Labeo rohita, Biocatal. Agricult. Biotech., 2020, vol. 25, p. 101582.
Uzo-God, O.C., Agarwal, A., and Singh, N.B., Effects of dietary nano and macro iron oxide (Fe2O3) on the growth, biochemical, and hematological profiles of African catfish (Clarias gariepinus) fingerlings, J. Appl. Aquacult., 2019, vol. 31, pp. 153–171.
Vangen, B. and Hemre, G.I., Dietary carbohydrate, iron and zinc interactions in Atlantic salmon (Salmo salar), Aquaculture, 2003, vol. 219, pp. 597–611.
Yousefian, M., Amiri, S., and Kor, D., Serum biochemical parameter of male, immature and female Persian sturgeon (Acipencer persicus), Astr. J. Bas. Appl. Res., 2011, vol. 5, pp. 476–481.
Zhang, H., Wang, H., Hu, K., Jiao, L., Zhao, M., Yang, X., and Xia, L., Effect of dietary supplementation of Lactobacillus casei YYL3 and L. plantarum YYL5 on growth, immune response and intestinal microbiota in channel catfish, Animals (Basel), 2019, vol. 9, pp. 1005–1020.
Ziaei-Nejad, S., Habibi Rezaei, M., Azari Takami, Gh., Lovett, L.D., Mirvaghefi, A.R., and Shakouri, M., The effect of Bacillus spp. bacteria used as probiotics on digestive enzyme activity, survival and growth in the Indian white shrimp Fenneropenaeus indicus, Aquaculture, 2006, vol. 252, pp. 516–524.
Ziaei-Nejad, S., Salehi, L.M., Ghaednia, B., Johari, S.A., and Aberomand, A., In vitro antagonistic properties of copper nanoparticles and probiotic Bacillus subtilis against pathogenic luminescent Vibrio harveyi, AACL Biol., 2015, vol. 8, pp. 445–452.
Ziaei-nejad, S., Shojaee, S.S., and Amini Chermahini, M., Effects of enriched Artemia with selenium nanoparticles on growth, survival and biochemical factors of guppy (Poecilia reticulata), Iran. J. Fish. Sci., 2020 (in press).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Ziaei-nejad, S., Abaei, N.K., Doost, B.N. et al. Effects of Supplemental Feeding of Common Carp (Cyprinus carpio) with Iron Nanoparticles and Probiotic Lactobacillus on Blood Biochemical Factors. Biol Bull Russ Acad Sci 48, 177–184 (2021). https://doi.org/10.1134/S1062359021020163
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359021020163