Abstract
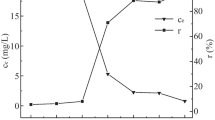
The agricultural waste, i.e., peanut husk, was modified with iron oxide employed in Cr(VI) removal from wastewater. In this study, standard biochar (BCPH) was prepared from the pyrolysis process from peanut husk. The prepared biochar was magnetized by impregnating the iron oxide onto the surface of the biochar to enhance the adsorption capacity of the adsorbent. The morphological analysis and physicochemical properties of the synthesized biochar (BCPH) and magnetic biochar (MBCPH) were evaluated systematically by SEM, XRD, BET surface area (SBET) and point of zero charge (pHZPC). Batch experiments were performed to evaluate the sorption mechanism and adsorption characteristics. The pH study revealed that maximum removal efficiency obtained in acidic condition (pHsolution < pHzpc) of the solution. The pseudo-second-order reaction model is employed to kinetic data to determine the equilibrium time, maximum removal efficacy, and the adsorption rate constants of BCPH and MBCPH. The equilibrium time obtained at 90 min for MBCPH and 150 min for BCPH, and the corresponding maximum removal efficiency values are 95.27% and 45.56%, respectively. The isotherm data corroborated that the Freundlich model is the best fit model for both adsorbents. The maximum adsorption capacities were found 8.51 mg/g for BCPH and 75.66 mg/g for MBCPH, respectively. The results show that the uptake capacity of the MBCPH is increased nine times compared to biochar (BCPH). The study concluded that the magnetic biochar (MBCPH) has higher uptake capacity and that can be used as a promising adsorbent for the separation of Cr(VI) from aqueous solution.













Similar content being viewed by others
Data Availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Zhu, S.; Huang, X.; Wang, D.; Wang, L.; Ma, F.: Enhanced hexavalent chromium removal performance and stabilization by magnetic iron nanoparticles assisted biochar in aqueous solution: mechanisms and application potential. Chemosphere 207, 50–59 (2018)
Cai, W.; Gu, M.; Jin, W.; Zhou, J.: CTAB-functionalized C@ SiO2 double-shelled hollow microspheres with enhanced and selective adsorption performance for Cr (VI). J. Alloy. Compd. 777, 1304–1312 (2019)
Cai, W.; Wei, J.; Li, Z.; Liu, Y.; Zhou, J.; Han, B.: Preparation of amino-functionalized magnetic biochar with excellent adsorption performance for Cr (VI) by a mild one-step hydrothermal method from peanut hull. Colloids. Surf. A. 563, 102–111 (2019)
Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q.: Mechanisms of metal sorption by biochars: biochar characteristics and modifications. Chemosphere 178, 466–478 (2017)
Son, E.B.; Poo, K.M.; Chang, J.S.; Chae, K.J.: Heavy metal removal from aqueous solutions using engineered magnetic biochars derived from waste marine macro-algal biomass. Sci. Total. Environ. 615, 161–168 (2018)
Dong, X.; Ma, L.Q.; Li, Y.: Characteristics and mechanisms of hexavalent chromium removal by biochar from sugar beet tailing. J. Hazard. Mater. 190(1–3), 909–915 (2011)
Agrafioti, E.; Kalderis, D.; Diamadopoulos, E.: Arsenic and chromium removal from water using biochars derived from rice husk, organic solid wastes and sewage sludge. J. Environ. Manag. 133, 309–314 (2014)
Han, Y.; Cao, X.; Ouyang, X.; Sohi, S.P.; Chen, J.: Adsorption kinetics of magnetic biochar derived from peanut hull on removal of Cr (VI) from aqueous solution: effects of production conditions and particle size. Chemosphere 145, 336–341 (2016)
Ma, Y.; Liu, W.J.; Zhang, N.; Li, Y.S.; Jiang, H.; Sheng, G.P.: Polyethylenimine modified biochar adsorbent for hexavalent chromium removal from the aqueous solution. Biores. Technol. 169, 403–408 (2014)
Pan, J.J.; Jiang, J.; Xu, R.K.: Removal of Cr (VI) from aqueous solutions by Na2SO3/FeSO4 combined with peanut straw biochar. Chemosphere 101, 71–76 (2014)
Evbuomwan, B.O.; Alalibo, J.T.: Proximate and ultimate analysis of walnut shell as a potential low cost adsorbent using different activating agents (KOH and H2SO4). Chem. Res. J. 2(5), 124–130 (2018)
Lowell, S.; Shields, J.E.: Powder Surface Area and Porosity, Vol. 2. Springer, New York (2013)
Kumar, R.; Mondal, S.: Removal of fluoride from aqueous solution using coal-coated with FeCl 3. In: Recent Developments in Waste Management, pp. 417–434. Springer, Singapore (2020)
Verma, L.; Siddique, M.A.; Singh, J.; Bharagava, R.N.: As (III) and As (V) removal by using iron impregnated biosorbents derived from waste biomass of Citrus limmeta (peel and pulp) from the aqueous solution and ground water. J. Environ. Manag. 250, 109452 (2019)
Langmuir, I.: The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 40(9), 1361–1403 (1918)
Freundlich, H.: Über die adsorption in lösungen. Z. Phys. Chem. 57(1), 385–470 (1907)
Temkin, M.J., Pyzhev, V. Recent modifications to Langmuir isotherms. (1940)
Dubinin, M. M. (1947). The equation of the characteristic curve of activated charcoal. In: Dokl. Akad. Nauk. SSSR, vol. 55, pp. 327–329
Garg, U.K.; Kaur, M.P.; Garg, V.K.; Sud, D.: Removal of hexavalent chromium from aqueous solution by agricultural waste biomass. J. Hazard. Mater. 140(1–2), 60–68 (2007)
Yang, X.; Al-Duri, B.: Kinetic modeling of liquid-phase adsorption of reactive dyes on activated carbon. J. Colloid. Interface. Sci. 287(1), 25–34 (2005)
Gupta, S.; Babu, B.V.: Removal of toxic metal Cr (VI) from aqueous solutions using sawdust as adsorbent: equilibrium, kinetics and regeneration studies. Chem. Eng. J. 150(2–3), 352–365 (2009)
Davarnejad, R.; Dastnayi, Z.K.; Kennedy, J.F.: Cr (VI) adsorption on the blends of Henna with chitosan microparticles: Experimental and statistical analysis. Int. J. Biol. Macromol. 116, 281–288 (2018)
Bansal, M.; Garg, U.; Singh, D.; Garg, V.K.: Removal of Cr (VI) from aqueous solutions using pre-consumer processing agricultural waste: a case study of rice husk. J. Hazard. Mater. 162(1), 312–320 (2009)
Kooh, M.R.R.; Dahri, M.K.; Lim, L.B.: Removal of the methyl violet 2B dye from aqueous solution using sustainable adsorbent Artocarpus odoratissimus stem axis. Appl. Water. Sci. 7(7), 3573–3581 (2017)
Mondal, S.; Mahanta, C.: Evaluation of arsenic adsorption capacity of indigenous materials for their suitability as filter media. Desalin. Water. Treat 84, 309–323 (2017)
Chien, S.H.; Clayton, W.R.: Application of Elovich equation to the kinetics of phosphate release and sorption in soils 1. Soil. Sci. Soc. Am. J. 44(2), 265–268 (1980)
Ho, Y.S.; McKay, G.: A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Trans. IChemE. 76(4), 332–340 (1998)
Ho, Y.S.; McKay, G.: Comparative sorption kinetic studies of dye and aromatic compounds onto fly ash. J. Environ. Sci. Health. Part. A. 34(5), 1179–1204 (1999)
Soliemanzadeh, A.; Fekri, M.: The application of green tea extract to prepare bentonite-supported nanoscale zero-valent iron and its performance on removal of Cr (VI): effect of relative parameters and soil experiments. Microporous. Mesoporous. Mater. 239, 60–69 (2017)
Zhao, H.; Lang, Y.: Adsorption behaviors and mechanisms of florfenicol by magnetic functionalized biochar and reed biochar. J. Taiwan. Inst. Chem. Eng. 88, 152–160 (2018)
Fan, Y.; Yang, R.; Lei, Z.; Liu, N.; Lv, J.; Zhai, S.; Wang, L.: Removal of Cr (VI) from aqueous solution by rice husk derived magnetic sorbents. Korean. J. Chem. Eng. 33(4), 1416–1424 (2016)
Vithanage, M.; Mayakaduwa, S.S.; Herath, I.; Ok, Y.S.; Mohan, D.: Kinetics, thermodynamics and mechanistic studies of carbofuran removal using biochars from tea waste and rice husks. Chemosphere 150, 781–789 (2016)
Njoku, V.O.; Islam, M.A.; Asif, M.; Hameed, B.H.: Preparation of mesoporous activated carbon from coconut frond for the adsorption of carbofuran insecticide. J. Anal. Appl. Pyrol. 110, 172–180 (2014)
Ma, H.; Yang, J.; Gao, X.; Liu, Z.; Liu, X.; Xu, Z.: Removal of chromium (VI) from water by porous carbon derived from corn straw: Influencing factors, regeneration and mechanism. J. Hazard. Mater. 369, 550–560 (2019)
Periyasamy, S.; Gopalakannan, V.; Viswanathan, N.: Fabrication of magnetic particles imprinted cellulose based biocomposites for chromium (VI) removal. Carbohyd. Polym. 174, 352–359 (2017)
Ahmad, M.; Lee, S.S.; Dou, X.; Mohan, D.; Sung, J.K.; Yang, J.E.; Ok, Y.S.: Effects of pyrolysis temperature on soybean stover-and peanut shell-derived biochar properties and TCE adsorption in water. Biores. Technol. 118, 536–544 (2012)
Aryee, A.A.; Mpatani, F.M.; Zhang, X.; Kani, A.N.; Dovi, E.; Han, R.; Li, Z.; Qu, L.: Iron (III) and iminodiacetic acid functionalized magnetic peanut husk for the removal of phosphate from solution: characterization, kinetic and equilibrium studies. J. Clean. Prod. (2020). https://doi.org/10.1016/j.jclepro.2020.122191
Lingamdinne, L.P.; Lee, S.; Choi, J.S.; Lebaka, V.R.; Durbaka, V.R.P.; Koduru, J.R.: Potential of the magnetic hollow sphere nanocomposite (graphene oxide-gadolinium oxide) for arsenic removal from real field water and antimicrobial applications. J. Hazard. Mater. (2021). https://doi.org/10.1016/j.jhazmat.2020.123882
Lingamdinne, L.P.; Choi, J.S.; Choi, Y.L.; Yang, J.K.; Koduru, J.R.; Chang, Y.Y.: Green activated magnetic graphitic carbon oxide and its application for hazardous water pollutants removal. Metals (2019). https://doi.org/10.3390/met9090935
Liu, Y.; Sohi, S.P.; Liu, S.; Guan, J.; Zhou, J.; Chen, J.: Adsorption and reductive degradation of Cr (VI) and TCE by a simply synthesized zero valent iron magnetic biochar. J. Environ. Manag. 235, 276–281 (2019)
Mishra, S.; Yadav, S.S.; Rawat, S.; Singh, J.; Koduru, J.R.: Corn husk derived magnetized activated carbon for the removal of phenol and para-nitrophenol from aqueous solution: Interaction mechanism, insights on adsorbent characteristics, and isothermal, kinetic and thermodynamic properties. J Environ. Manag. (2019). https://doi.org/10.1016/j.jenvman.2019.06.013
Funding
This work was supported by grants from the Department of Science & Technology and Biotechnology, Government of West Bengal (Memo No. 231(Sanc.)/ST/P/S&T/5G-19/2017 dated 24/03/2018).
Author information
Authors and Affiliations
Contributions
Byomkesh Mahanty performed the batch study, kinetic study, isotherm study, thermodynamic study, interpreted the rate of reaction and types of adsorption and was a major contributor in writing the manuscript. Sandip Mondal analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Mahanty, B., Mondal, S. Synthesis of Magnetic Biochar Using Agricultural Waste for the Separation of Cr(VI) From Aqueous Solution. Arab J Sci Eng 46, 10803–10818 (2021). https://doi.org/10.1007/s13369-021-05572-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-021-05572-0